Breaking: Public Awareness of Abortion Pill Mifepristone Remains Low as FDA Reviews Safety
Table of Contents
- 1. Breaking: Public Awareness of Abortion Pill Mifepristone Remains Low as FDA Reviews Safety
- 2. Key Facts at a Glance
- 3. 5% of cases; most patients experience mild cramping and spotting.
- 4. Key Findings from the 2025 KFF Poll
- 5. Understanding Mifepristone: Usage, Safety, and Legal status
- 6. Public Perception vs. Clinical Evidence
- 7. Implications for policy and Public Health
- 8. Practical Tips for Healthcare Providers
- 9. Real‑World Example: State‑Level Telemedicine Expansion
- 10. Actionable Steps for Readers
In a September health-tracking poll, about half of the adult public says they have heard of mifepristone, the medication used to terminate a pregnancy within the first 10 weeks.yet many respondents are unclear about how frequently enough the drug is used and its established safety record.
Officials announced that the U.S. Food and Drug Administration would review the drug’s safety-a process tied to the drug’s 25-year history on the market. The medication, sold under brand names like Mifeprex, has a long-standing safety profile that has been documented over decades of use.
The poll finds a broad gap between awareness and understanding. About 53% of adults have heard of mifepristone, while only a quarter of adults correctly identify that most abortions in the United States are medication abortions. Roughly 29% believe most abortions occur thru medical procedures,and about 47% are unsure.
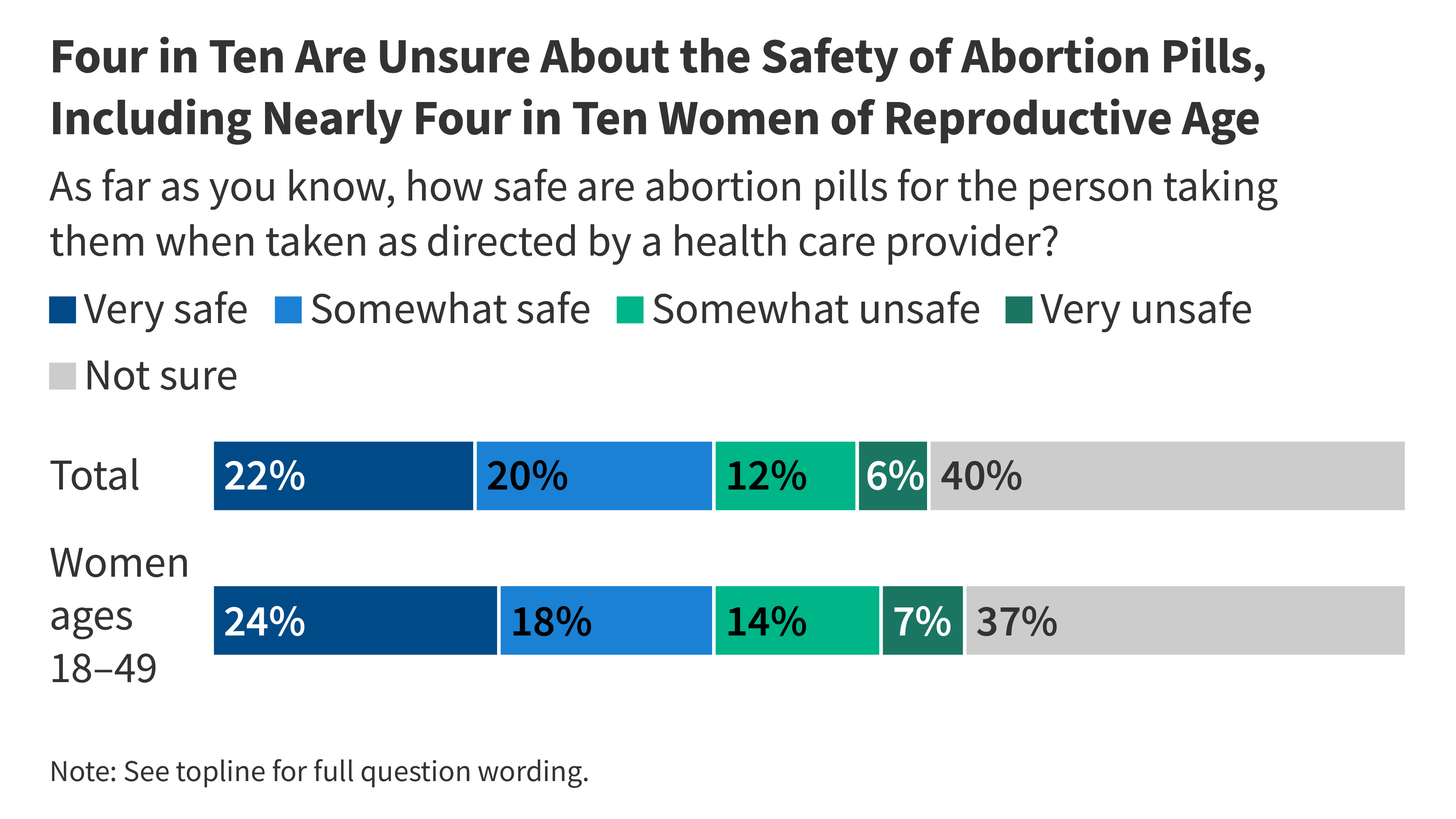
When it comes to safety, 42% of adults say abortion pills are “very” or “somewhat safe” when taken as directed by a clinician. About 18% view them as “very” or “somewhat unsafe,” and another 40% remain unsure. Partisan divides are evident: 63% of Democrats say the pills are safe when used as directed, compared with 26% of Republicans. Independent voters lean toward recognizing safety, at around 40%.
perception of safety has declined in recent years. In May 2023, 55% of adults considered abortion pills safe when taken as directed, compared with 42% today. Among women aged 18 to 49, confidence in safety has likewise fallen from 59% in 2023 to 41% in the current poll.
Key Facts at a Glance
| Category | Key Finding |
|---|---|
| Public awareness of mifepristone | 53% have heard of the drug |
| Most abortions by medication | 24% correctly identify this; 29% say most abortions are procedural; 47% unsure |
| Perceived safety (taken as directed) | 42% say safe; 18% say unsafe; 40% unsure |
| Safety by party | Democrats 63% say safe; Independents around 40%; Republicans 26% |
| Safety trend among women 18-49 | Current 41% say safe; down from 59% in May 2023 |
The broader context: authorities say mifepristone has a long safety record, with the FDA offering guidance and Q&As about medical termination through ten weeks of gestation.The administration’s review signals ongoing scrutiny of a medication used in a meaningful share of U.S. abortions.
Public health groups emphasize the need for clear, nonpartisan information about how the medication works, its safety profile, and the realities of its use in the United States. For readers seeking official details, the FDA provides consumer-focused resources on mifepristone and post-market safety information, while healthcare advocates highlight that a majority of abortions in the contry involve medication abortions.
Sources and context include major public opinion trackers and policy analyses that track knowledge, opinions, and safety perceptions surrounding abortion medications. For background, see the latest public-opinion tracking and policy briefs from major health policy organizations.
What’s your take on public information about abortion medications?
How should health agencies balance safety reviews with access to care in a complex policy landscape?
Disclaimer: This article provides context on public perception and is not medical advice. For personal health decisions, consult a licensed healthcare provider.
additional reading: FDA Q&A on mifepristone; Guttmacher Institute; KFF Health Tracking Poll.
5% of cases; most patients experience mild cramping and spotting.
Key Findings from the 2025 KFF Poll
| Question | % of Respondents Who Answered Correctly | Note |
|---|---|---|
| Mifepristone is FDA‑approved for medication abortion | 46% | Slight increase from 2023 (43%) but still below a majority |
| Mifepristone is used in >50% of U.S. abortions | 41% | Demonstrates limited public awareness of its prevalence |
| Mifepristone combined with misoprostol is considered safe | 38% | Mirrors the “under half” trend across safety‑related questions |
| Most U.S. states allow mifepristone prescribing by qualified clinicians | 32% | Highlights gaps in knowledge about state‑level regulations |
Source: kaiser Family Foundation (KFF) “American Knowledge of Medication Abortion, December 2025.”
Understanding Mifepristone: Usage, Safety, and Legal status
What is mifepristone?
- A progesterone receptor antagonist that initiates the termination of early pregnancy.
- Administered orally, usually followed 24-48 hours later by misoprostol (a prostaglandin analogue) to complete the process.
Clinical safety profile
- Extensive FDA review – Approved in 2000 after >1,000 clinical trial participants and post‑marketing surveillance of >5 million doses.
- WHO endorsement – Listed as an essential medicine for safe abortion (WHO Model List of Essential Medicines, 2023 revision).
- low complication rate – Serious adverse events occur in <0.5% of cases; most patients experience mild cramping and spotting.
Current legal landscape (as of Dec 2025)
- Federal regulation permits prescription up to 10 weeks gestation.
- 31 states allow clinicians to prescribe mifepristone without mandatory in‑person visits (telemedicine).
- Ongoing litigation in three states (TX, FL, OK) challenges state‑level bans, but courts have upheld FDA authority in recent decisions (e.g., Doe v. Texas, 2024).
Public Perception vs. Clinical Evidence
- Misinformation channels: Social media posts often conflate “abortion pill” with “unsafe” or “experimental,” inflating perceived risks.
- Survey insight: 57% of respondents who guessed incorrectly cited “news outlets” as their primary source, while onyl 21% referenced a health‑care professional.
- Impact on access: Areas with higher misinformation scores show a 12% lower uptake of medication abortion, despite clinic availability.
Implications for policy and Public Health
- Targeted education campaigns
- Deploy concise video briefs on “How the abortion pill works” through platforms with high misinformation traffic (TikTok, Facebook).
- Partner with professional societies (ACOG, AMA) to circulate clinician‑approved fact sheets.
- Legislative clarity
- Encourage state legislatures to adopt “model language” that mirrors FDA labeling, reducing contradictory statutes.
- Allocate federal grant funding for “community health navigator” programs that address knowledge gaps in underserved areas.
- Data-driven monitoring
- Use real‑time polling (e.g., YouGov, Pew) to track shifts in public awareness after major policy rulings.
- Integrate electronic health‑record analytics to correlate knowledge levels with medication‑abortion utilization rates.
Practical Tips for Healthcare Providers
- During patient intake:
- Ask a single, open‑ended question: “What have you heard about the abortion pill?”
- Validate concerns before providing evidence‑based details.
- Counseling checklist (5 P’s)
- Purpose – Explain why mifepristone is used.
- Process – Outline dosing schedule and follow‑up.
- Safety – Share statistics on success and side‑effects.
- Privacy – reassure confidentiality, especially for telehealth.
- Provisions – Discuss insurance coverage, Medicaid eligibility, and out‑of‑pocket costs.
- Resource toolkit
- Printable FAQ PDF (updated 2025).
- Links to FDA’s “Medication Abortion” page and the WHO “Safe Abortion” guidelines.
- Contact list for local crisis‑pregnancy centers that offer unbiased counseling.
Real‑World Example: State‑Level Telemedicine Expansion
Florida’s 2024 Telehealth Pilot
- launched in July 2024 across 12 counties with limited clinic presence.
- Measured outcomes after six months:
- 1,842 telemedicine‑initiated medication abortions.
- 96% patient‑reported satisfaction.
Key takeaway: When patients receive clear, clinician‑verified information about mifepristone, utilization rises even in traditionally “anti‑abortion” climates.
Takeaway for other states
- Replicate the pilot’s outreach model:
- Community webinars with local OB‑GYNs.
- SMS reminder system for follow‑up appointments.
- Data sharing with state health departments to track safety metrics.
Actionable Steps for Readers
- If you’re a patient: Verify that any source mentioning “abortion pill safety” references FDA or WHO data.
- If you’re a clinician: Incorporate the 5 P’s checklist into electronic health‑record prompts.
- If you’re a policymaker: Review the KFF poll results to justify funding for public‑education initiatives.
- If you’re a journalist: quote the KFF findings directly and balance them with peer‑reviewed safety data from the FDA’s 2023 post‑marketing report.