Here’s a breakdown of what the article is about and what you can learn from it:
Summary of the Article:
Table of Contents
- 1. Summary of the Article:
- 2. What You Can Learn from This Article:
- 3. What are the potential cardiac consequences of abruptly stopping heart failure medications, as highlighted in the text?
- 4. HF Drug Benefit Diminishes Rapidly Following Cessation
- 5. understanding the Transient Effects of Heart failure Medications
- 6. The Physiological Basis for Benefit Loss
- 7. Timeline of Benefit Diminution: what to Expect
- 8. Implications for Patient Management & Discontinuation Strategies
- 9. Real-World Example: The Case of
This article reports on a secondary analysis of the FINEARTS-HF trial, investigating the consequences of discontinuing the medication finerenone in patients wiht heart failure (HF). While finerenone showed benefits during the trial in reducing serious cardiovascular adverse events and deaths, its abrupt withdrawal after long-term use was associated with a importent increase in these events, particularly those related to HF itself. This suggests that finerenone, like other HF therapies, may have lasting benefits and should not be stopped without careful consideration.
What You Can Learn from This Article:
- the Risks of Stopping Finerenone in Heart Failure: The primary takeaway is that abruptly stopping finerenone after long-term use can be hazardous. it significantly increases the risk of serious cardiovascular events and death, with an observed 2.8-fold increase in such events compared to continuing the medication.
- The Importance of Treatment Persistence in Heart Failure: The study reinforces the general guideline that HF therapies are often recommended for lifelong use due to their presumed lasting benefits. It challenges the idea that stable HF means medications can be safely discontinued.
- Finerenone’s Specific Impact on HF: The article highlights that the increased events following finerenone withdrawal were predominantly HF-specific, underscoring the drug’s direct role in managing the condition.
- Clinical Implications for Patients and Doctors:
For Clinicians: The researchers advise against automatically interrupting MRA (mineralocorticoid receptor antagonist) therapy, like finerenone, due to minor changes in potassium or kidney function. This suggests a need for careful risk-benefit assessments before stopping treatment.
For Patients: Patients with HF on finerenone should discuss any concerns about continuing the medication with their doctor, rather than stopping it on their own.
- Methodology of the Study: You can learn about how the researchers conducted this analysis, including:
It was a secondary analysis of a larger trial (FINEARTS-HF).
The study involved withdrawing the intervention (finerenone or placebo) in surviving patients.
Blinding was maintained during the withdrawal phase.
adverse events were monitored for a specific period (approximately 30 days post-withdrawal).
- limitations of the Study: It’s critically important to note the study’s weaknesses, such as:
The possibility of patient comparability issues years after randomization.
Reliance on adverse event reporting without adjudication, which might affect accuracy.
* The low number of events during the withdrawal period, limiting statistical precision.
- Funding and Disclosures: The article clarifies the funding source (Bayer) and financial disclosures of the researchers, which is standard practice for scientific publications.
In essence, this article serves as a crucial warning about the potential dangers of discontinuing finerenone in heart failure patients and emphasizes the need for continued treatment adherence to maintain stability and prevent serious adverse outcomes.
What are the potential cardiac consequences of abruptly stopping heart failure medications, as highlighted in the text?
HF Drug Benefit Diminishes Rapidly Following Cessation
understanding the Transient Effects of Heart failure Medications
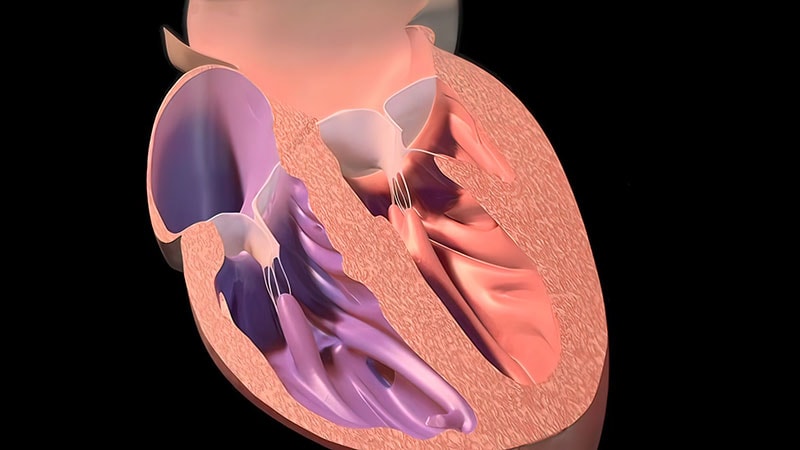
Heart failure (HF) medications are crucial for managing symptoms and improving quality of life for individuals living with this chronic condition. However, a critical aspect often underestimated is the rapid decline in benefits observed after discontinuing thes drugs. This isn’t a simple “stopping medication” scenario; it’s a complex physiological response demanding careful consideration.This article explores why the benefits of HF drugs diminish so quickly,the implications for patient management,and strategies to mitigate risks. We’ll focus on common medications like ACE inhibitors, ARBs, beta-blockers, and diuretics, and their impact on heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).
The Physiological Basis for Benefit Loss
The positive effects of HF medications aren’t merely symptomatic relief; they induce significant physiological changes. These changes, while beneficial during treatment, are often not sustained upon cessation.
Reverse Remodeling: Many HF drugs, particularly ACE inhibitors, ARBs, and beta-blockers, promote reverse remodeling of the heart. This means reducing the size of the enlarged heart chambers and improving the heart muscle’s ability to contract. This remodeling isn’t permanent. When the medication is stopped, the heart gradually reverts to its previous, enlarged state.
Neurohormonal Reactivation: HF triggers activation of the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system. Medications target these systems to reduce strain on the heart. Stopping these drugs leads to rapid reactivation of these neurohormonal pathways, increasing blood pressure, heart rate, and fluid retention – all detrimental to heart failure.
Reduced Diuretic Effect: Diuretics reduce fluid overload, alleviating symptoms like shortness of breath and edema. Without continued diuretic therapy, fluid accumulates, leading to a swift return of congestion.
Loss of Beta-Blocker Protection: Beta-blockers not only slow heart rate but also protect the heart from the damaging effects of adrenaline. Removing this protection allows for increased cardiac workload and potential arrhythmias.
Timeline of Benefit Diminution: what to Expect
The speed at which benefits diminish varies depending on the specific medication and the individual patient,but a general timeline can be observed:
Diuretics (1-3 days): Fluid retention begins almost immediately. Patients often report noticeable weight gain and worsening edema within days.
ACE inhibitors/ARBs (1-2 weeks): Neurohormonal reactivation starts within a week, leading to rising blood pressure and potential worsening of heart failure symptoms. Reverse remodeling begins to reverse.
Beta-blockers (2-4 weeks): the protective effects against arrhythmias and excessive heart rate diminish over several weeks. Patients may experience palpitations or increased shortness of breath.
Mineralocorticoid Receptor Antagonists (MRAs) – (2-6 weeks): Similar to ACEi/ARBs, neurohormonal reactivation and fluid retention contribute to symptom recurrence.
Implications for Patient Management & Discontinuation Strategies
Understanding this rapid decline is crucial for effective patient management. Abrupt discontinuation of HF medications is rarely advisable.
Gradual Tapering: When possible, medications should be tapered slowly under close medical supervision. This allows the body to adjust and minimizes the risk of acute decompensation.
Close Monitoring: Patients undergoing medication changes require frequent monitoring of weight, blood pressure, heart rate, and symptoms.
Identifying the Reason for Discontinuation: The reason for stopping a medication is paramount. Is it due to side effects, intolerance, or a perceived improvement in condition? This dictates the approach to discontinuation.
Alternative Therapies: If a medication needs to be stopped due to side effects, exploring alternative therapies within the same drug class or different classes is essential.
* Patient Education: Patients must be thoroughly educated about the potential consequences of stopping their medications and the importance of adhering to the prescribed regimen.