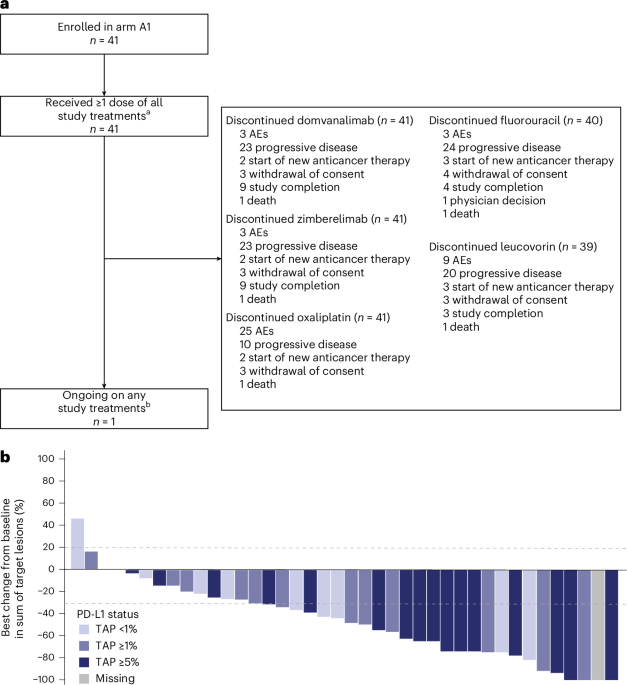
Gastric Cancer Treatment Breakthrough: The Dawn of TIGIT and PD-1 Combination Therapy
A staggering 80% of gastric cancers are diagnosed at advanced stages, drastically reducing survival rates. But a recent Phase 2 trial, presented at the ESMO Congress 2025 and published in Nature Medicine, is shifting that paradigm. The combination of domvanalimab (an Fc-silent anti-TIGIT antibody) and zimberelimab (an anti-PD-1 antibody) alongside standard FOLFOX chemotherapy is demonstrating remarkably promising results in first-line treatment for advanced gastric and gastroesophageal cancers, hinting at a future where these aggressive cancers are far more manageable.
Unlocking the Immune System: How TIGIT and PD-1 Blockade Work
For years, immunotherapy has revolutionized cancer treatment, but not all patients respond. The key lies in overcoming the mechanisms cancers use to evade the immune system. **Gastric cancer** often expresses proteins that suppress immune cell activity. PD-1, a protein on immune cells, acts as a brake, preventing them from attacking cancer cells. Anti-PD-1 therapies, like zimberelimab, release this brake. However, some cancers develop resistance. This is where TIGIT comes in.
TIGIT is another protein on immune cells that, when activated, further dampens the immune response. Domvanalimab, an Fc-silent anti-TIGIT antibody, blocks TIGIT, providing a second layer of immune activation. The “Fc-silent” aspect is crucial; it minimizes unintended immune reactions, potentially improving safety. By simultaneously blocking both PD-1 and TIGIT, this combination therapy aims to unleash a more robust and sustained anti-cancer immune response.
Beyond Response Rates: The Significance of Survival Outcomes
While objective response rates (the percentage of patients whose tumors shrink or disappear) are important, they don’t tell the whole story. The Phase 2 trial showed encouraging response rates, but the real excitement stems from the observed improvements in progression-free survival (PFS) and overall survival (OS). These metrics indicate how long patients live without their cancer worsening and how long they live overall, respectively. These early survival data are compelling enough to warrant a larger, Phase 3 confirmatory trial, which is already planned.
The Future of Gastric Cancer Treatment: What to Expect
This trial isn’t just about domvanalimab and zimberelimab; it’s a signal of a broader trend. Researchers are increasingly focused on combining different immunotherapies to overcome resistance and maximize efficacy. We’re likely to see more clinical trials exploring combinations of anti-TIGIT antibodies with other immune checkpoint inhibitors, as well as with chemotherapy and targeted therapies.
One critical area of investigation will be identifying biomarkers – measurable characteristics in a patient’s tumor or blood – that predict who is most likely to benefit from this combination therapy. Currently, predicting response to immunotherapy remains a challenge. Biomarker discovery will allow for a more personalized approach, ensuring that the right patients receive the right treatment at the right time. The National Cancer Institute provides a comprehensive overview of immunotherapy.
Personalized Medicine and the Role of Genomic Profiling
The future of gastric cancer treatment will undoubtedly involve a deeper understanding of the genomic landscape of each patient’s tumor. Genomic profiling can reveal specific mutations and alterations that drive cancer growth and identify potential vulnerabilities. This information can be used to tailor treatment strategies, potentially combining immunotherapy with targeted therapies that address specific genetic abnormalities. The integration of genomic data with immunotherapy response data will be crucial for optimizing treatment outcomes.
Implications for Other Cancers
The success of this TIGIT/PD-1 combination in gastric cancer has implications beyond the gastrointestinal tract. TIGIT is expressed in various other cancers, including non-small cell lung cancer, melanoma, and bladder cancer. The principles learned from this trial – the potential of dual immune checkpoint blockade and the importance of identifying predictive biomarkers – can be applied to these other malignancies. We may see similar combination therapies being investigated in these settings, offering hope to patients with a wider range of cancers.
The data from this Phase 2 trial represent a significant step forward in the fight against gastric cancer. While the Phase 3 trial is essential to confirm these findings, the early results are undeniably encouraging, suggesting a future where this aggressive disease is no longer a death sentence. What are your predictions for the role of TIGIT inhibitors in the broader cancer immunotherapy landscape? Share your thoughts in the comments below!