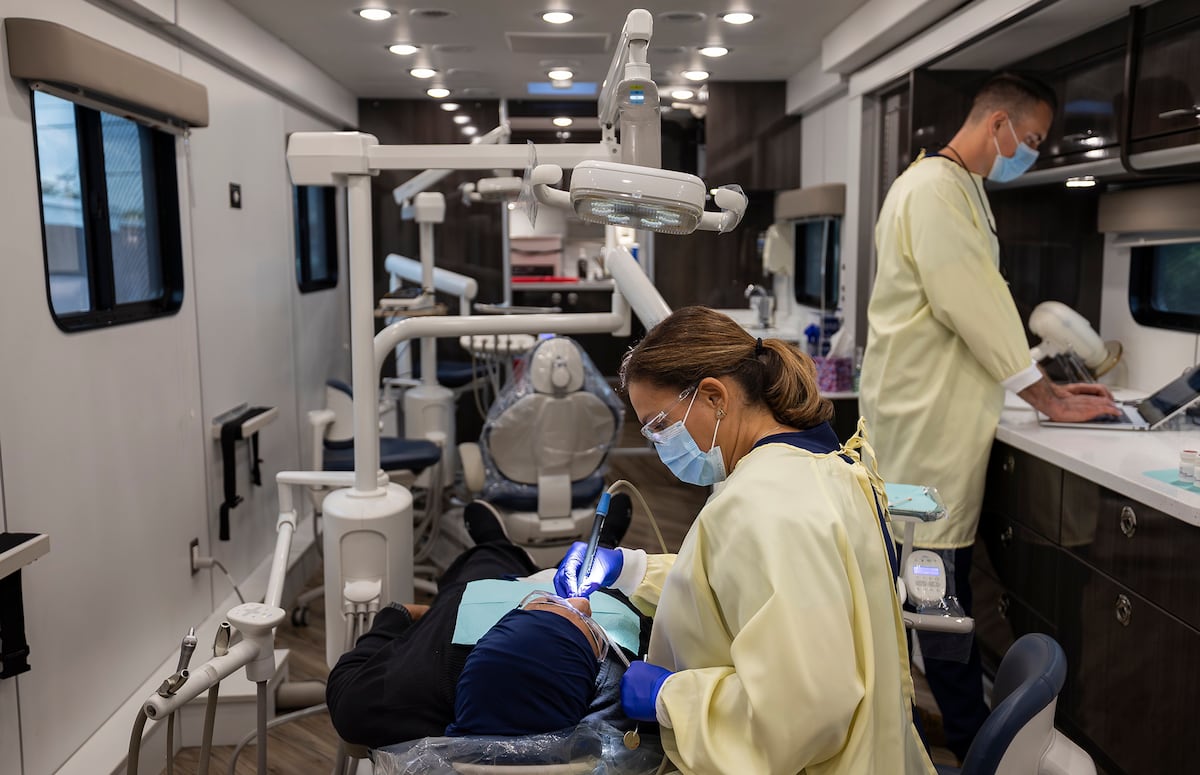
The promise of artificial intelligence to revolutionize healthcare delivery faces a significant hurdle: successful implementation. A recent large-scale trial involving an AI-enabled stethoscope for detecting cardiovascular disease demonstrated the technology’s potential, but ultimately revealed that low adoption rates and workflow disruptions significantly limited its real-world effectiveness. The findings underscore the complexities of integrating even highly effective AI tools into existing healthcare systems.
Early detection of cardiovascular diseases like heart failure, atrial fibrillation, and valvular heart disease is critical for improving patient outcomes. However, primary care settings often lack the resources for comprehensive screening. AI-powered stethoscopes, which analyze electrocardiogram and phonocardiogram signals, have emerged as a potential solution for point-of-care detection. But translating promising research results into tangible improvements in patient care is proving to be a complex undertaking.
TRICORDER Trial: A Promising Technology Faces Real-World Obstacles
The study, known as TRICORDER (Triple Cardiovascular Disease Detection using an Artificial Intelligence Stethoscope), was a pragmatic, cluster-randomized controlled trial conducted within the UK’s National Health Service (NHS). It represents the largest deployment of cardiovascular AI within the NHS to date, according to researchers in the European Heart Journal. While the AI stethoscope demonstrated an ability to enhance the detection of these conditions when used as intended, the trial revealed significant challenges in getting clinicians to consistently utilize the technology.
Researchers found that despite the AI stethoscope’s accuracy, uptake among healthcare professionals was lower than anticipated. Workflow integration proved difficult, hindering the tool’s ability to deliver on its potential. These implementation gaps ultimately hampered the overall effectiveness of the trial. The study details are available on ClinicalTrials.gov, identifying it as an open-label, cluster randomised controlled trial (NCT05987670).
The Importance of Incentivization and Workflow Integration
The challenges highlighted in the TRICORDER trial aren’t unique to this specific technology. Experts suggest that successful AI implementation requires careful consideration of how novel tools fit into existing clinical workflows and the provision of adequate incentives for adoption. The Nature article explains that for a newly introduced AI tool, even one proven effective, to be sustainably adopted, the role of incentivization is crucial.
The findings align with broader concerns about the “implementation gap” in healthcare AI. A study published in The Lancet demonstrates that AI-enabled stethoscopes offer robust performance characteristics in point-of-care detection of heart failure, atrial fibrillation, and valvular heart disease, but real-world impact depends on consistent leverage.
Looking Ahead: Bridging the Gap Between Innovation and Implementation
The TRICORDER trial serves as a valuable lesson for the future of AI in healthcare. Simply developing effective AI tools isn’t enough; healthcare systems must proactively address the logistical and behavioral challenges associated with their implementation. Future research and deployment strategies should prioritize user-centered design, workflow integration, and appropriate incentivization to maximize the benefits of AI for both clinicians and patients.
The path forward requires a collaborative effort involving technology developers, healthcare providers, and policymakers to ensure that the promise of AI in cardiovascular disease detection – and in healthcare more broadly – is fully realized. What strategies will be most effective in overcoming these implementation hurdles remains a key question for ongoing research.
Share your thoughts on the challenges of implementing AI in healthcare in the comments below.
Disclaimer: This article provides informational content and should not be considered medical advice. Always consult with a qualified healthcare professional for diagnosis and treatment of any medical condition.