The Obesity Debate Isn’t About Calories – It’s About Hormones, and Research is Finally Catching Up
For decades, the prevailing wisdom has been that weight gain is simply a matter of “calories in, calories out.” But a growing body of research, spearheaded by scientists like Dr. David Ludwig, is challenging this long-held belief. New evidence suggests that carbohydrate-insulin model of obesity – the idea that hormonal responses to different foods, particularly carbohydrates, play a crucial role in regulating body weight – is gaining traction, and could fundamentally change how we approach diet and health. This isn’t just academic debate; it has profound implications for everything from public health policy to your next meal.
The Flaws in the Calorie-Centric View
The “calories in, calories out” model assumes that all calories are created equal, and that weight regulation is solely determined by the balance between energy intake and expenditure. However, this fails to account for the complex physiological processes that govern hunger, metabolism, and fat storage. As Ludwig and colleagues have demonstrated, restricting calories can often lead to metabolic slowdown, increased hunger, and ultimately, weight regain. This is because calorie restriction impacts hormones, particularly insulin, which plays a key role in fat storage.
Insulin’s Role: Beyond Blood Sugar
The carbohydrate-insulin model posits that consuming refined carbohydrates and sugars leads to a surge in insulin levels. Chronically elevated insulin doesn’t just manage blood sugar; it actively promotes fat storage and inhibits fat burning. This creates a vicious cycle where more carbohydrates lead to more insulin, leading to more fat storage, and increased hunger. This contrasts sharply with the effects of fat and protein, which elicit a smaller insulin response and promote satiety.
The Mounting Evidence: From Metabolic Ward Studies to Real-World Observations
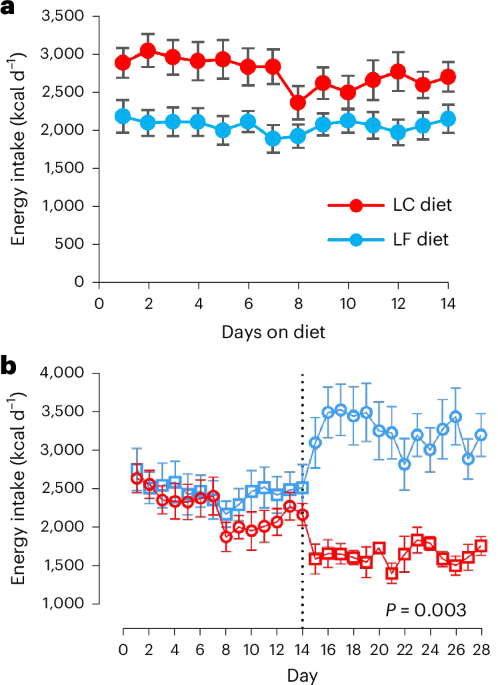
Initial support for the carbohydrate-insulin model came from studies showing that individuals on high-carbohydrate diets tend to overeat, while those on high-fat diets naturally reduce their calorie intake. More recent research, including a study by Hall et al. (2021) comparing plant-based, low-fat diets to animal-based, ketogenic diets, initially seemed to contradict this, showing similar calorie intake across groups. However, the debate continues, with concerns raised about the limitations of short-term dietary trials and the impact of physiological adaptation to macronutrient changes, as highlighted by Soto-Mota et al. (2024).
The Problem with Crossover Trials: Carryover Effects
A critical issue complicating dietary research is the phenomenon of “carryover effects.” As Ludwig, Willett, and Putt (2025) explain, when participants cycle through different diets in a crossover trial, the physiological effects of one diet can linger and influence their response to the next. This can distort results and lead to inaccurate conclusions. For example, adapting to a high-carbohydrate diet can alter insulin sensitivity, impacting how the body responds to a subsequent low-carbohydrate diet. The ability to detect these carryover effects requires careful study design and statistical power, often lacking in shorter trials (Putt, 2006; Senn, 1988).
Diet Order Matters: New Findings on Energy Balance
Recent work by Sciarrillo et al. (2024) further complicates the picture, demonstrating that the order in which diets are presented in crossover studies significantly affects energy balance. Participants consumed more calories after a high-carbohydrate diet compared to after a high-fat diet, even when macronutrient composition was controlled. This reinforces the idea that metabolic adaptation and hormonal responses are crucial factors, and that simply measuring calorie intake isn’t enough.
Beyond Obesity: The Broader Health Implications
The implications of this shifting understanding extend beyond weight management. Emerging research suggests that dietary carbohydrate levels can influence inflammation and immune function. A study by Link et al. (2024) found that vegan and ketogenic diets elicited distinct peripheral immune signatures, highlighting the potential for diet to modulate immune responses. This suggests that the carbohydrate-insulin model may have relevance to a wide range of chronic diseases, including heart disease, type 2 diabetes, and even autoimmune conditions.
The Future of Nutritional Science: Personalized Approaches and Long-Term Studies
The ongoing debate between the energy balance and carbohydrate-insulin models isn’t about proving one side “right” and the other “wrong.” It’s about recognizing the limitations of simplistic models and embracing a more nuanced understanding of human metabolism. The future of nutritional science likely lies in personalized approaches that consider individual metabolic profiles, hormonal responses, and genetic predispositions. Longer-term studies, designed to minimize carryover effects and account for physiological adaptation, are crucial. We need to move beyond short-term calorie counting and focus on the quality of food and its impact on our hormonal environment. Ultimately, understanding the interplay between carbohydrates, insulin, and metabolism is key to unlocking lasting health and well-being.
What dietary changes have you found most effective for managing your weight and health? Share your experiences in the comments below!