Recent advancements in biomarker research have highlighted the potential of DOPA decarboxylase (DDC) as a diagnostic tool for Lewy body disorders, which include conditions like dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD). Researchers have developed a quantitative immunoassay to measure DDC levels in cerebrospinal fluid (CSF), showing promise in differentiating between DLB, Alzheimer’s disease (AD), and other related disorders.
The study involved participants from six different cohorts, including clinical validation groups, and was conducted in adherence to ethical guidelines, including the Declaration of Helsinki. All participants or their legal representatives provided informed consent for the use of their biological data in research.
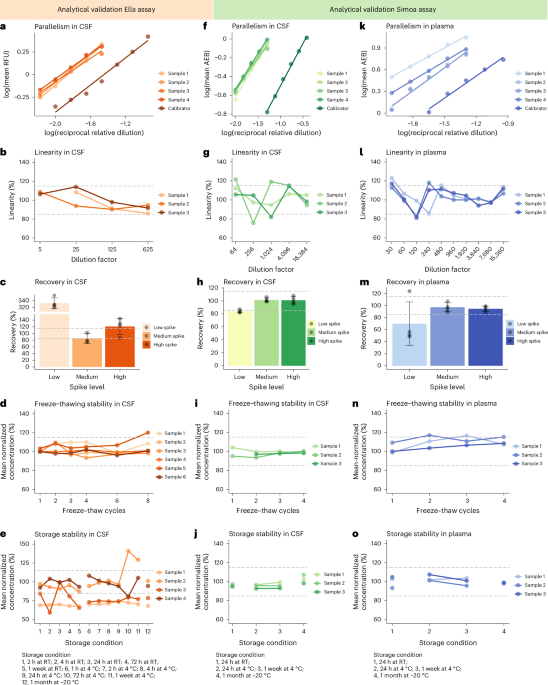
Utilizing platforms such as SimplePlex Ella and Quanterix Simoa, researchers optimized a sandwich enzyme-linked immunosorbent assay (ELISA) for detecting DDC concentrations in CSF, plasma, and serum. This methodology was validated for crucial parameters like lower limits of detection and precision, establishing a robust framework for its application in clinical settings.
Study Population and Methodology
The study successfully enrolled participants from various cohorts, including the ADC Discovery cohort, which consisted of 50 controls, 41 DLB patients, and 51 patients with Alzheimer’s disease. The multicenter validation cohort further expanded the sample size, incorporating additional diagnostic groups such as mild cognitive impairment (MCI) and other dementias.
Core CSF biomarkers, including amyloid-beta (Aβ42), phosphorylated tau (pTau181), and total tau (tTau), were used to support clinical diagnoses of AD and to confirm underlying amyloid pathology. These biomarkers were measured using established commercial assays, ensuring the reliability of the diagnostic process.
Quantitative Measurement and Analytical Validation
The DDC levels in CSF were measured using both the Ella and Simoa platforms. Notably, samples were subjected to various analytical validations, including assessments of dilution linearity and sample stability. The acceptable coefficients of variation were set below 20%, ensuring high accuracy in the results. The researchers employed statistical methods to analyze DDC concentrations across different diagnostic groups, revealing significant distinctions between DLB and other dementias.
In particular, the DDC assay demonstrated a strong potential to distinguish DLB from Parkinson’s disease and other neurodegenerative disorders. Receiver operating characteristic (ROC) analyses were performed to determine the assay’s efficacy, with results suggesting that DDC levels could serve as a reliable biomarker for identifying DLB in clinical practice.
Immunohistochemical Findings
immunohistochemistry was utilized to assess DDC presence in postmortem brain tissue from patients diagnosed with DLB and PDD. This analysis revealed significant DDC expression in specific brain regions, including the substantia nigra and raphe nucleus, areas commonly affected in Lewy body pathologies. This correlational study supports the hypothesis that elevated DDC levels may be indicative of underlying neurodegenerative processes.
The researchers found that combining DDC measurements with established biomarkers could enhance diagnostic accuracy, offering a comprehensive approach to identifying Lewy body disorders. This development marks a significant step towards personalized medicine, where early and accurate diagnosis can lead to better patient management.
Implications and Future Directions
The findings from this study underscore the importance of DDC as a potential diagnostic biomarker for Lewy body disorders. As research progresses, there is hope that DDC measurements may become part of routine clinical assessments, aiding in the differentiation of complex neurodegenerative disorders.
Moving forward, continued validation of DDC as a biomarker in larger, diverse populations will be critical. Integrating DDC measurements with advanced imaging techniques and other biomarkers may provide deeper insights into the pathophysiology of Lewy body disorders. Such advancements could also pave the way for targeted therapies and improved patient outcomes.
As the field of neurodegenerative research evolves, the potential for biomarkers like DDC to redefine diagnostic criteria offers a promising outlook for future clinical practice. Stakeholders in the healthcare sector are encouraged to engage in discussions about these developments and share their thoughts on the implications for patient care.
Disclaimer: This article is for informational purposes only and does not constitute medical advice.