AI Offers New Hope for Critical Care in Under-Resourced Hospitals
Table of Contents
- 1. AI Offers New Hope for Critical Care in Under-Resourced Hospitals
- 2. Predicting Neurological Recovery After Cardiac Arrest
- 3. the Need for AI governance and Ethical Frameworks
- 4. Introducing POLARIS-GM
- 5. AI in Healthcare: A Comparative Look
- 6. The Challenge: Diagnostic Gaps in Underserved Areas
- 7. Singapore Deploys AI to Enhance Diagnostics in Low‑Resource Settings
- 8. The Challenge: Diagnostic Gaps in Underserved Areas
- 9. AI-Powered Solutions: A Deep Dive
- 10. Singapore’s Collaborative Ecosystem
- 11. Real-World Impact & Case Studies
- 12. Benefits of AI in Low-Resource Diagnostics
- 13. Practical Tips for Implementation
January 31, 2026
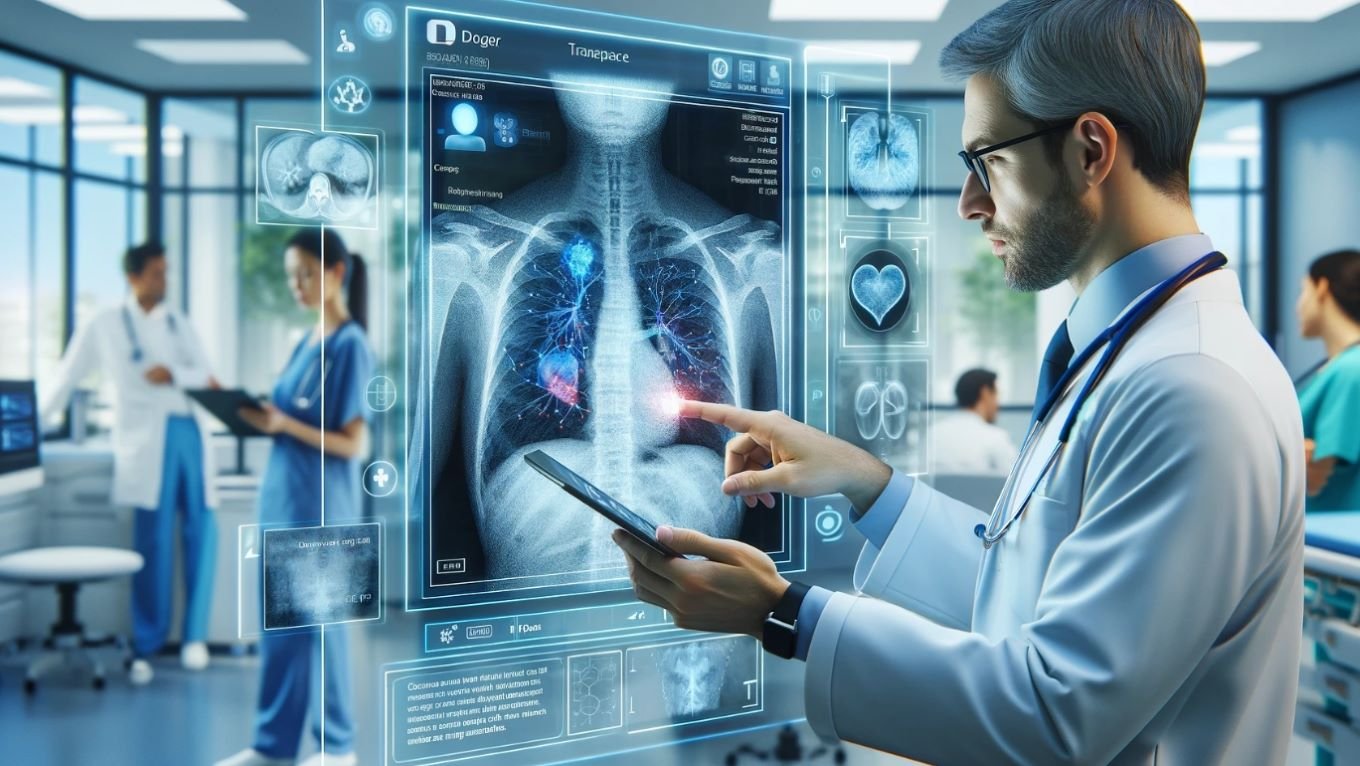
Singapore is leading the charge in leveraging Artificial Intelligence to revolutionize diagnostics, particularly in healthcare settings grappling with limited resources. The innovative approach promises to improve patient outcomes and streamline critical decision-making where access to specialized tools and extensive datasets is frequently enough restricted. This is a pivotal development as healthcare disparities persist globally.
Predicting Neurological Recovery After Cardiac Arrest
Following a cardiac arrest, determining a patient’s potential for neurological recovery is a complex and often agonizing process. Access to complex diagnostic equipment, like advanced brain imaging, can be severely limited in manny hospitals, especially those in lower- and middle-income countries. Researchers at Duke-NUS Medical School, collaborating with international partners, have developed and adapted an advanced Artificial Intelligence model to accurately forecast neurological recovery in such environments.
The team employed a technique called transfer learning, wich allows an AI model initially trained on large, complete datasets to be effectively repurposed for new scenarios with comparatively limited local data. This method circumvents the need for extensive data collection, making it practical and cost-effective for broader implementation. recent data from the World Health institution indicates that over 75% of deaths from cardiovascular disease occur in low- and middle-income countries, highlighting the urgent need for accessible, affordable diagnostic tools.
the Need for AI governance and Ethical Frameworks
While the potential benefits of AI in healthcare are considerable, experts stress the importance of robust governance frameworks to ensure safe and ethical deployment.Current regulations governing medical technologies often struggle to address the unique risks associated with AI, including data privacy, the potential for algorithmic bias, and the challenge of assigning accountability when AI systems make incorrect predictions.Concerns about “model hallucinations” – where AI generates plausible but inaccurate information – are also paramount.
Introducing POLARIS-GM
To proactively address these challenges, researchers from Duke-NUS have spearheaded the creation of an international consortium named the Partnership for Oversight, Leadership, and Accountability in Regulating Bright Systems-Generative Models in Medicine (POLARIS-GM). This initiative aims to establish actionable best practices for regulating AI tools, continuously monitoring their impact, and creating safety measures that are particularly suited to resource-constrained settings.
The consortium’s objectives include developing clear guidelines for data usage, transparency in algorithmic decision-making, and mechanisms for identifying and mitigating potential biases. It also seeks to establish protocols for ongoing monitoring and evaluation to ensure AI systems remain accurate and reliable over time. A study published in npj Digital Medicine underscored the importance of continuous validation of AI models in real-world clinical settings.
AI in Healthcare: A Comparative Look
| Feature | Conventional Diagnostics | AI-Assisted Diagnostics |
|---|---|---|
| Cost | High (equipment, expertise) | Lower (reduced need for specialized personnel) |
| Accessibility | Limited in resource-poor settings | Potentially widespread with cloud-based solutions |
| Speed | Variable, often time-consuming | Faster, real-time analysis possible |
| Accuracy | Dependent on expert interpretation | Potentially higher with reduced human error |
The advancement of AI in healthcare isn’t just about technological innovation; it’s about global health equity. By bridging the gap in diagnostic capabilities, AI has the potential to save lives and improve the quality of care for millions.
What steps do you believe are moast critical for ensuring the ethical and responsible implementation of AI in healthcare? And how can we overcome the barriers to adoption in under-resourced regions?
Share your thoughts in the comments below, and don’t forget to share this article with your network!
The Challenge: Diagnostic Gaps in Underserved Areas
Singapore Deploys AI to Enhance Diagnostics in Low‑Resource Settings
Singapore is rapidly becoming a global leader in leveraging artificial intelligence (AI) to address healthcare challenges, notably in improving diagnostic capabilities within low-resource settings. This initiative isn’t just about technological advancement; it’s a strategic move to enhance global health equity and accessibility. The focus extends beyond the nation’s borders, aiming to create scalable solutions for regions facing limitations in specialist access, infrastructure, and trained personnel.
The Challenge: Diagnostic Gaps in Underserved Areas
Many developing nations struggle with notable diagnostic gaps. These gaps stem from several interconnected factors:
* Shortage of Specialists: Limited numbers of radiologists, pathologists, and other diagnostic specialists.
* Uneven Infrastructure: Lack of access to advanced medical imaging equipment like MRI, CT scanners, and even reliable digital pathology systems.
* Geographical Barriers: Remote populations face difficulties accessing centralized diagnostic facilities.
* Cost Constraints: High costs associated with specialist consultations and advanced diagnostic tests.
These challenges lead to delayed or inaccurate diagnoses, ultimately impacting patient outcomes and increasing healthcare burdens. AI-powered diagnostic tools offer a promising pathway to bridge these gaps.
AI-Powered Solutions: A Deep Dive
Singapore’s approach centers on developing and deploying AI algorithms capable of analyzing medical images and data with high accuracy, even in the absence of highly trained specialists. Several key areas are seeing significant progress:
1. AI-Assisted Radiology:
* Automated Image Analysis: AI algorithms can automatically detect anomalies in X-rays, CT scans, and MRIs, flagging potential issues for review by clinicians. This is particularly valuable in detecting conditions like tuberculosis, pneumonia, and certain cancers.
* Reduced Reporting Time: AI can pre-populate radiology reports, significantly reducing the workload for radiologists and accelerating diagnosis.
* Improved Accuracy: Studies have shown AI can achieve diagnostic accuracy comparable to, and in certain specific cases exceeding, that of human radiologists, especially in identifying subtle patterns.
2. Digital Pathology & AI:
* Whole Slide Imaging (WSI): Digitizing pathology slides allows for remote consultation and AI-powered analysis.
* Cancer Detection & Grading: AI algorithms are being trained to identify cancerous cells and accurately grade tumors, assisting pathologists in making precise diagnoses. This is crucial for personalized treatment planning.
* Reduced Turnaround Time: AI can prioritize slides for pathologist review, speeding up the diagnostic process.
3. Point-of-Care Diagnostics with AI:
* Smartphone-Based diagnostics: AI-powered apps are being developed to analyze images captured by smartphone cameras, enabling rapid diagnosis of skin conditions, eye diseases, and other ailments in remote areas.
* Portable Ultrasound with AI Guidance: AI can assist healthcare workers with performing and interpreting ultrasound scans, even without extensive training.
* Rapid Disease Screening: AI algorithms can analyze data from simple diagnostic tests (e.g., blood tests, urine analysis) to quickly identify individuals at risk of specific diseases.
Singapore’s Collaborative Ecosystem
A key factor driving Singapore’s success is its robust collaborative ecosystem. This includes:
* Government Support: Significant investment from the Singaporean government in AI research and growth, particularly through initiatives like Smart Nation.
* Public-Private Partnerships: Collaboration between hospitals, research institutions (e.g., National University of Singapore, Nanyang Technological University), and private companies specializing in AI and healthcare.
* Data Sharing & Standardization: efforts to establish standardized datasets and data sharing protocols to facilitate AI model training and validation.
* regulatory Frameworks: Development of clear regulatory guidelines for the deployment of AI-powered medical devices, ensuring patient safety and data privacy.
Real-World Impact & Case Studies
While still evolving, several initiatives demonstrate the potential of singapore’s approach:
* National Tuberculosis Screening Program: AI algorithms are being used to analyze chest X-rays for signs of tuberculosis, improving screening efficiency and early detection rates.
* Diabetic Retinopathy Screening: AI-powered systems are automating the screening process for diabetic retinopathy, a leading cause of blindness, in primary care settings.
* Collaboration with Southeast Asian Nations: Singapore is actively partnering with countries like Indonesia, Vietnam, and the Philippines to deploy AI-powered diagnostic tools and provide training to local healthcare professionals. A notable example is the pilot program in rural Vietnam utilizing AI-assisted ultrasound for prenatal care.
Benefits of AI in Low-Resource Diagnostics
The benefits extend far beyond improved accuracy and efficiency:
* Increased Access to Care: Brings diagnostic capabilities to underserved populations.
* Reduced healthcare Costs: Early and accurate diagnosis can prevent costly complications and hospitalizations.
* Empowered Healthcare Workers: provides support and guidance to healthcare professionals with limited training.
* Improved Patient Outcomes: Leads to faster treatment and better overall health outcomes.
* Scalability & Sustainability: AI solutions can be scaled to meet the needs of large populations and are relatively sustainable in the long term.
Practical Tips for Implementation
Successfully deploying AI in low-resource settings requires careful planning and execution:
- Focus on Specific Needs: Identify the most pressing diagnostic challenges in the target region.
- data Quality is Paramount: Ensure access to high-quality, representative datasets for AI model training.
- User-Centric Design: Develop AI tools that are easy to use and integrate seamlessly into existing workflows.
- Training & Capacity Building: Provide thorough training to healthcare professionals on how to use and interpret AI-powered diagnostic results.
- Continuous Monitoring & Evaluation: Regularly monitor the performance of AI algorithms and make adjustments as needed.
- Address Ethical Considerations: Ensure data privacy, algorithmic fairness, and openness in