Demographic characteristics of the study population
Table of Contents
- 1. Demographic characteristics of the study population
- 2. Occurrence of HCV viremia
- 3. Genotype distribution among the study population
- 4. Phylogenetic analysis of the study
- 5. Assessment of DAA therapy
- 6. Clinical assessment before and after DAA treatment
- 7. Liver related adverse events
- 8. ## Summary of “Acting Antiviral Treatment Outcomes for Chronic Hepatitis C Across Various Genotypes in West Bengal, India”
- 9. Real-World Insights into Direct-Acting antiviral Treatment Outcomes for Chronic Hepatitis C Across Various Genotypes in West Bengal, India
- 10. understanding Hepatitis C Prevalence in West Bengal
- 11. The Revolution of Direct-Acting Antivirals (DAAs)
- 12. Real-World Treatment Outcomes: A Genotype-Specific Analysis
- 13. Genotype 3: The Dominant Challenge
- 14. Genotype 1: Achieving High SVR with Optimized Regimens
- 15. Genotype 6: Emerging Data and Treatment Strategies
- 16. Factors Influencing Treatment Success Beyond Genotype
- 17. Addressing Barriers to Access and Affordability
- 18. Monitoring and Post-Treatment Follow-Up
- 19. benefits of Successful HCV Treatment
- 20. Practical Tips for Healthcare Professionals
This study comprised 254 HCV sero-reactive individuals with 133 males (52.36%) and 121 females (47.63%). The age of the study population ranged from 20 to 83 years with a mean age of 51.91(± 11.82) years. The mean viral load of the RNA-positive individuals was 6.06(± 6.18) log IU/ml, and no significant difference in viral load was observed between genotype 3 and genotype 1.
Occurrence of HCV viremia
The frequency of active HCV infection in this study population was evaluated. Overall, HCV RNA positivity was 58.26%. HCV viremia was observed among 54.14% and 62.81% of males and females, respectively. The study found that HCV RNA positivity was highest (66.67%) in people aged 52 to 59. Blood transfusion, medical procedures, and improper use of syringes/needles were the common risk factors associated with active infection in the study, with blood transfusion accounting for the majority of patients (68.83%) (p < 0.05). Of the total 254 patients, 110 were cirrhotic and 144 non-cirrhotic. Among the cirrhotic patients, 69 (62.7%) were having decompensated cirrhosis. Decompensated cirrhosis of liver was associated with HCV viremia in 72.46% of cases (p < 0.05) (Table 1).
Genotype distribution among the study population
Two HCV genotypes and seven subtypes were predominating in this study population (Fig- 1b). Of the 148 individuals that tested positive for HCV RNA, 120 (81%) had infection with genotype-3 (3a- 50%, 3b- 46.67%, 3 g- 2.5%, and 3i- 0.83%), while 28 (19%) had infection with genotype-1 (1a- 21.43%, 1b- 67.86%, and 1c- 10.71%). When the prevalence of HCV genotypes was compared across the seven age categories, those between the ages of 52 and 59 were most likely to have genotype 3 infection (82.22%, n = 37) (p < 0.05). Genotype distribution across gender, mode of transmission and status of cirrhosis is listed in Table 2.
Phylogenetic analysis of the study
A phylogenetic tree was constructed with the representative sequences obtained from this study population (Supplementary Fig – 2) with reference sequences from the NCBI database. Corresponding representative sequences were found to cluster with the respective reference sequences.
The phylogenetic tree was constructed by using MEGA X software package with 34 reference sequences downloaded from NCBI and 65 representative sequences from in-house sequencing. Prefix “NICED-VL/CLD” denoted in-house sequences.
Assessment of DAA therapy
This study monitored DAA therapy in 148 HCV RNA-positive individuals for 48 weeks. Of these 148 individuals, 120 individuals infected with genotype 3 were treated with Sofosbuvir (SOF) + Daclatasvir (DCV), and 28 individuals infected with genotype 1 were offered Sofosbuvir (SOF) + Ledipasvir (LDV)[[19, 20]. After 4 weeks of treatment, individuals were followed up for assessing the attainment of RVR, 8 patients (5.40%) (Male = 3, Female = 5) had lost follow-up within the first month of DAA treatment and the rest (n = 140) showed 100% RVR. During the 12-week follow-up, 15 patients (10.71%) (Male = 7, Female = 8) had lost follow-up. Out of the remaining 125 patients, 4 patients (3.2%) tested positive again, and remaining 121 patients showed EVR (96.8%). In the next follow-up visit, 112 patients (92.56%) showed SVR24while 9 patients (7.44%) tested RNA positive again. Hence, the SVR24 achievement rate was 92.56% (Fig 2).
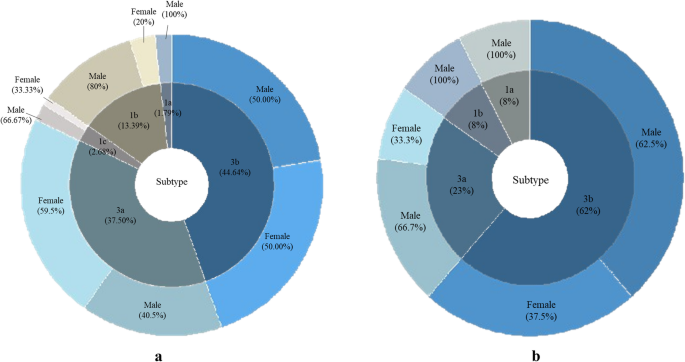
Out of 112 patients who achieved SVR2458 (51.78%) were male and 54(48.21%) were female. Gender-wise HCV genotype distribution of these 112 patients was gen-3a (42, 37.5%) (Female- 59.5% and male- 40.5%), gen-3b (50, 44.64%) (Female- 50% and male- 50%), gen-1a (2, 1.79%) (Male- 100%), gen-1b (15, 13.39%) (Female- 20% and male- 80%), and gen-1c (3, 2.68%) (Female- 33.33% and male- 66.67%) (Fig 3a) and among 13 relapse patients, 9 (69.24%) were male and 4 (30.76%) were female. Genotypically, these 13 relapse patients were distributed as follows- gen-3a (3, 23%) (Female- 33.3% and male- 66.7%), gen-3b (8, 62%) (Female- 37.5% and male- 62.5%), gen-1a (1, 7.69%) (Male- 100%), and gen 1b (1, 7.69%) (Male-100%) (Fig 3b).
Patients (n = 13) who failed to achieve SVR24 (relapsed) were re-interviewed and counseled again. A modified treatment regime with SOF + velpatasvir (VEL) + ribavirin (Riba) was prescribed to everyone re-interviewed for an additional 6 months. Of the 13 relapse patients taking SOF + VEL + Riba for another 24 weeks, 12 patients (92.3%) did not show active infection (considered as SVR’24 achieved), and in contrast, 1 patient (7.69%) continued to be nonresponsive to DAA. Overall, 99.2% of patients achieved either SVR24 or SVR’24. Patients who have achieved SVR24 were requested to revisit after 48 weeks for SVR48 evaluation. At the SVR48 assessment, only 65 patients (58.04%) attended, and upon evaluating the status of viremia, undetectable HCV RNA was found in all patients. Therefore, it can be inferred that among the attended patients the SVR48 achievement rate is 100%. Fig-2 represents the treatment follow-up for 48 weeks.
Furthermore, Supplementary Fig-3 provides an overview of DAA efficacy by bifurcating the study population on the basis of the status of cirrhosis. Of the 77 SVR achieved patients, 57.14% were cirrhotic and 42.86% were non-cirrhotic, while out of 70 lost follow-up cases, 34.29% were cirrhotic and 65.71% were non-cirrhotic.
Clinical assessment before and after DAA treatment
Liver parameters were assessed for any significant changes between the baseline and post-treatment condition. We found that ALP, SGPT and Total Bilirubin returned to normal post-treatment. Significant differences (p < 0.001) were observed between two conditions in ALP, SGOT, SGPT, albumin, globulin and total bilirubin levels (Table 3).
Among the 65 patients who completed 48 weeks of follow up, 37 were cirrhotic and 28 were non cirrhotic. Liver related adverse events were observed in 2 non-cirrhotic and 17 cirrhotic patients. Hepatomegaly and portal hypertension were the most common adverse effects. Cirrhotic patients had higher incidence of portal hypertension (13.5%) and hepatomegaly (13.5%). Occurrence of ascites (8.1%), hepatic encephalopathy (2.7%) and hepatocellular carcinoma (8.1%) were majorly observed in cirrhotic patients Table 4.
understanding Hepatitis C Prevalence in West Bengal
West Bengal, India, carries a important burden of Hepatitis C Virus (HCV) infection. Factors contributing to this include historical intravenous drug use, inadequate sterilization practices in healthcare settings, and blood transfusions before widespread screening. Determining the prevalent HCV genotypes is crucial for effective treatment strategies. Common genotypes in the region include genotype 3, followed by genotypes 1 and 6. Accurate HCV genotype testing is the first step towards personalized treatment.
The introduction of Direct-Acting Antivirals (DAAs) has dramatically altered the landscape of chronic Hepatitis C treatment.unlike older interferon-based therapies, DAAs offer:
* High Sustained Virologic Response (SVR) rates: Typically exceeding 95% across most genotypes.
* Shorter treatment durations: Usually 8-12 weeks, improving patient adherence.
* Fewer side effects: Leading to better patient tolerance and quality of life.
* Oral administration: Making treatment more convenient.
commonly used DAAs include sofosbuvir, daclatasvir, ledipasvir, and velpatasvir, often used in combination regimens. The choice of regimen depends on the HCV genotype, presence of cirrhosis, and prior treatment history.
Real-World Treatment Outcomes: A Genotype-Specific Analysis
Analyzing treatment outcomes across different genotypes in West Bengal reveals nuanced patterns.
Genotype 3: The Dominant Challenge
Genotype 3 is the most prevalent in West Bengal. Studies show consistently high SVR rates (96-98%) with regimens like sofosbuvir/daclatasvir for 12 weeks. However, challenges remain:
* Fibrosis Progression: A significant proportion of patients present with advanced fibrosis or cirrhosis at diagnosis, requiring careful monitoring.
* Treatment Adherence: Socioeconomic factors can impact adherence, potentially lowering SVR rates.
* Re-infection Risk: Individuals engaging in high-risk behaviors remain susceptible to re-infection.
Genotype 1: Achieving High SVR with Optimized Regimens
While less common than genotype 3, genotype 1 requires specific DAA combinations. Sofosbuvir/ledipasvir for 12 weeks demonstrates excellent efficacy (94-97%). Resistance-associated substitutions (RAS) are less frequent in genotype 1,simplifying treatment decisions.
Genotype 6: Emerging Data and Treatment Strategies
Genotype 6 is increasingly identified in West Bengal. Data on DAA efficacy for genotype 6 is still evolving, but preliminary results suggest that sofosbuvir-based regimens are generally effective. Ongoing research is crucial to refine treatment protocols.
Factors Influencing Treatment Success Beyond Genotype
Several factors beyond genotype impact HCV treatment outcomes:
- Liver Disease Severity: Patients with cirrhosis have lower SVR rates and a higher risk of complications.
- HIV co-infection: HIV/HCV co-infected individuals may require longer treatment durations or option regimens.
- diabetes and Metabolic Syndrome: These comorbidities can reduce treatment efficacy.
- Alcohol Consumption: Continued alcohol use negatively impacts treatment response.
- Drug Use: Active intravenous drug use increases the risk of re-infection.
- Treatment Adherence: Consistent medication intake is paramount for success.
Addressing Barriers to Access and Affordability
Despite the availability of DAAs,access remains a challenge for many in West bengal.
* Cost of Treatment: While generic DAAs have reduced costs, they are still unaffordable for some patients. Government subsidies and charitable programs are vital.
* Limited diagnostic Facilities: Access to HCV RNA testing and genotype testing is unevenly distributed.
* Lack of Awareness: Many individuals are unaware of their HCV status and do not seek testing or treatment.
* Stigma: Social stigma associated with HCV can deter individuals from seeking care.
Monitoring and Post-Treatment Follow-Up
Achieving SVR doesn’t guarantee complete resolution. Long-term monitoring is essential:
* Surveillance for Hepatocellular Carcinoma (HCC): Patients with cirrhosis require regular HCC screening.
* Assessment of Liver Fibrosis: Monitoring fibrosis progression or regression post-treatment.
* Monitoring for Re-infection: Especially in individuals with ongoing risk factors.
* Evaluation of Extrahepatic Manifestations: HCV can cause complications beyond the liver, requiring ongoing assessment.
benefits of Successful HCV Treatment
Successful HCV eradication offers significant benefits:
* Reduced risk of cirrhosis and liver failure.
* Decreased risk of HCC.
* Improved liver function.
* Enhanced quality of life.
* Reduced transmission risk.
Practical Tips for Healthcare Professionals
* Prioritize HCV screening in high-risk populations.
* Ensure accurate genotype testing before initiating treatment.
* select appropriate DAA regimens based on genotype