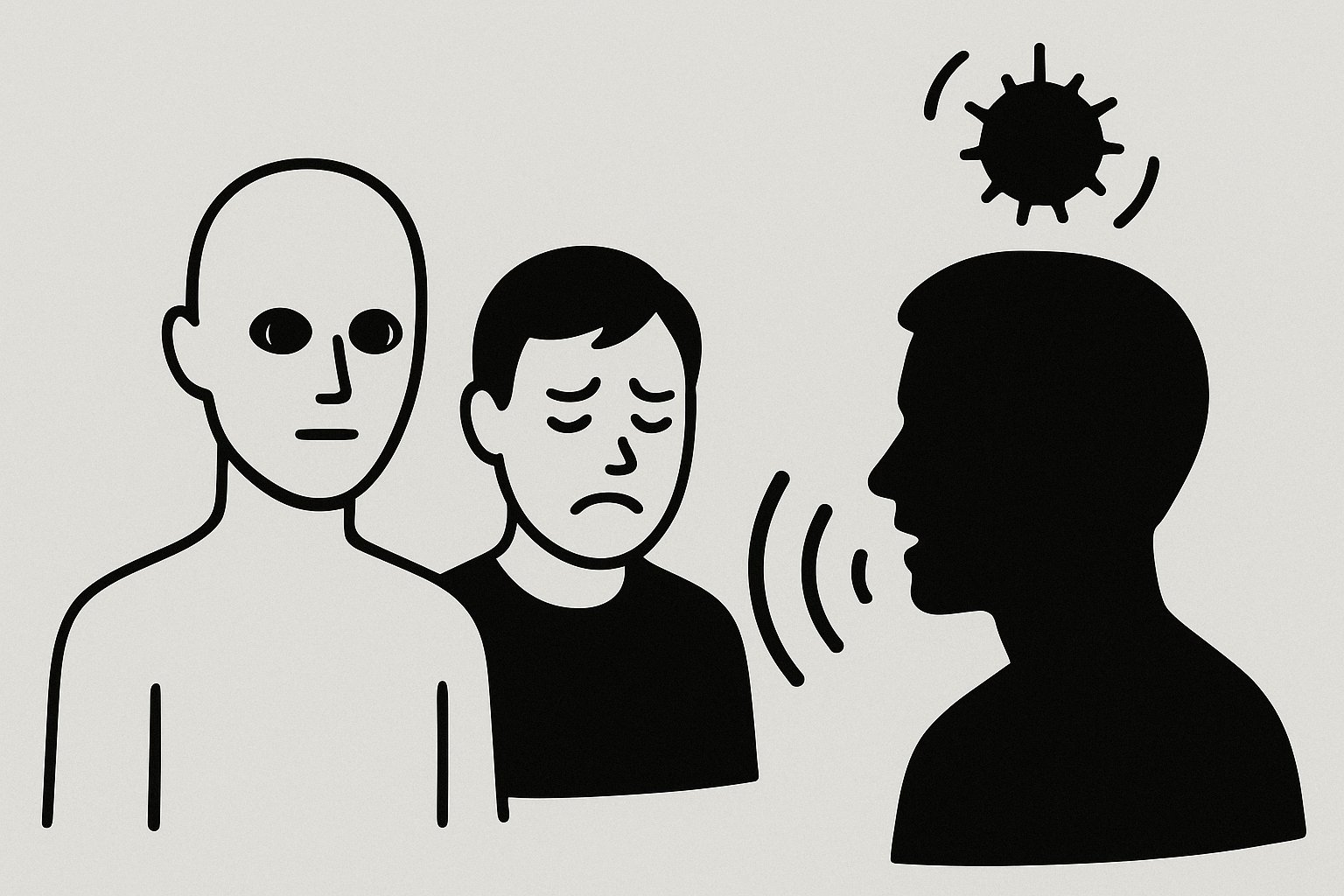
The Uncanny Immune Response: How ‘Almost Human’ Avatars Are Triggering Our Defenses
Imagine feeling inexplicably uneasy around a digital character – not because of its actions, but simply because something feels off about its appearance. Recent research from the University of Hamburg suggests this discomfort isn’t just psychological. It’s triggering a measurable, automatic immune response. This isn’t just about aesthetics; it’s about deeply ingrained survival mechanisms and a future where our bodies may be reacting to the digital world in ways we’re only beginning to understand.
The Biology of Discomfort: Unpacking the Study
The “uncanny valley” – that unsettling feeling we get when encountering entities that appear almost, but not quite, human – has long been a topic of debate in robotics, animation, and virtual reality. The Hamburg study took this a step further, demonstrating a physiological link. Researchers exposed participants to three types of virtual agents: cartoonish figures, highly realistic avatars, and avatars with subtle imperfections designed to fall into the uncanny valley. Crucially, they measured immunoglobulin A (sIgA) levels in participants’ saliva before and after interaction. sIgA is an antibody vital for immune function in mucous membranes, acting as a first line of defense against pathogens.
The results were striking. Only interaction with the disturbing, uncanny avatars led to a significant increase in sIgA concentration. This suggests that the brain isn’t just registering visual discomfort; it’s initiating an immune response, as if preparing for a potential threat. Even more fascinating, this response occurred unconsciously – participants weren’t necessarily aware of *why* they felt uneasy, yet their bodies were reacting defensively.
The Pathogen Avoidance Hypothesis and Digital Threats
Researchers believe this response is rooted in our evolutionary history. The “pathogen avoidance hypothesis” posits that humans have evolved to detect subtle cues indicating potential illness in others. These cues – slight asymmetries, unusual skin tones, or unnatural movements – might signal the presence of disease. Our brains, honed by millennia of survival, automatically activate protective mechanisms. In the context of avatars, these subtle imperfections, even if unintentional, could be misinterpreted as signs of illness, triggering the immune response.
Pro Tip: When designing virtual characters, prioritize subtle realism over striving for perfect human replication. Embrace stylistic choices that avoid the uncanny valley, focusing on clear communication and emotional expression rather than photorealistic detail.
Future Implications: Beyond Avatar Design
The implications of this research extend far beyond improving avatar aesthetics. As virtual and augmented reality become increasingly integrated into our lives, understanding this unconscious immune response is critical. Consider these potential future trends:
- Personalized Medicine & VR Therapy: If avatars used in therapeutic settings (e.g., exposure therapy for phobias, social skills training) trigger an immune response, it could hinder the therapeutic process. Careful avatar design will be paramount.
- The Metaverse and Social Anxiety: Prolonged exposure to uncanny avatars in immersive virtual environments could potentially exacerbate social anxiety or even contribute to chronic stress.
- AI Companions & Emotional Bonding: As AI companions become more sophisticated and visually realistic, the uncanny valley effect could impact our ability to form genuine emotional connections.
- Remote Healthcare & Telepresence: The design of remote healthcare interfaces, including robotic doctors or virtual nurses, must prioritize trust and comfort. An uncanny appearance could undermine patient confidence.
“Did you know?” that the uncanny valley effect isn’t limited to visual stimuli? Researchers are exploring similar responses to uncanny voices and movements, suggesting a broader sensory basis for this phenomenon.
The Rise of ‘Comfortable’ Digital Humans
We’re likely to see a shift towards designing digital humans that prioritize “approachability” over “realism.” This might involve embracing stylized aesthetics, focusing on expressive animation, and carefully controlling the level of detail. Companies like Epic Games, with their MetaHuman Creator, are already grappling with these challenges, offering tools to create realistic avatars but also emphasizing the importance of artistic control.
Furthermore, advancements in AI-driven animation could allow avatars to exhibit more natural and nuanced movements, reducing the subtle imperfections that trigger the uncanny valley effect. Expect to see algorithms that analyze human motion and replicate it with greater fidelity, creating more believable and comfortable digital interactions.
Expert Insight: “The key isn’t necessarily to eliminate the uncanny valley entirely, but to understand *why* it exists and to design digital characters that navigate it effectively. Sometimes, a slightly stylized or cartoonish appearance can be more engaging and trustworthy than a hyperrealistic one.” – Dr. Eleanor Vance, Cognitive Psychologist specializing in Human-Computer Interaction.
Actionable Insights for Developers and Designers
So, what can developers and designers do to mitigate the uncanny immune response? Here are a few key takeaways:
Embrace Stylization: Don’t be afraid to deviate from perfect realism. Stylized avatars can be just as engaging and avoid the pitfalls of the uncanny valley.
Iterative Testing: Conduct thorough user testing with diverse groups to identify and address any uncanny elements in your designs. Pay attention to both conscious feedback and physiological responses (e.g., heart rate, skin conductance).
Focus on Natural Movement: Invest in high-quality motion capture and animation techniques to create realistic and fluid movements.
Frequently Asked Questions
Q: Is the uncanny valley effect universal?
A: While the effect is widely observed, its intensity can vary across cultures and individuals. Exposure to technology and prior experiences may also play a role.
Q: Does this immune response have any long-term health consequences?
A: Currently, there’s no evidence to suggest long-term health consequences. However, further research is needed to understand the potential effects of prolonged exposure to uncanny avatars.
Q: Can the uncanny valley effect be overcome entirely?
A: It’s unlikely to be eliminated completely. However, by understanding the underlying mechanisms and applying thoughtful design principles, we can minimize its impact and create more comfortable and engaging digital experiences.
What are your predictions for the future of human-avatar interaction? Share your thoughts in the comments below!