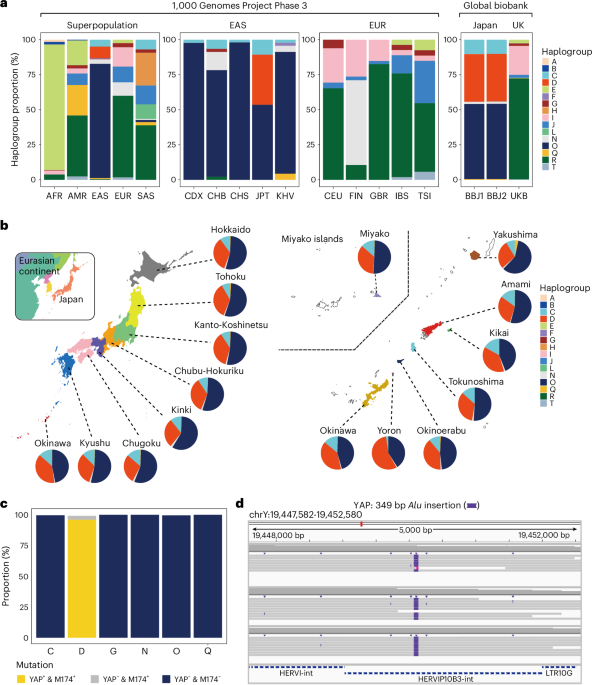
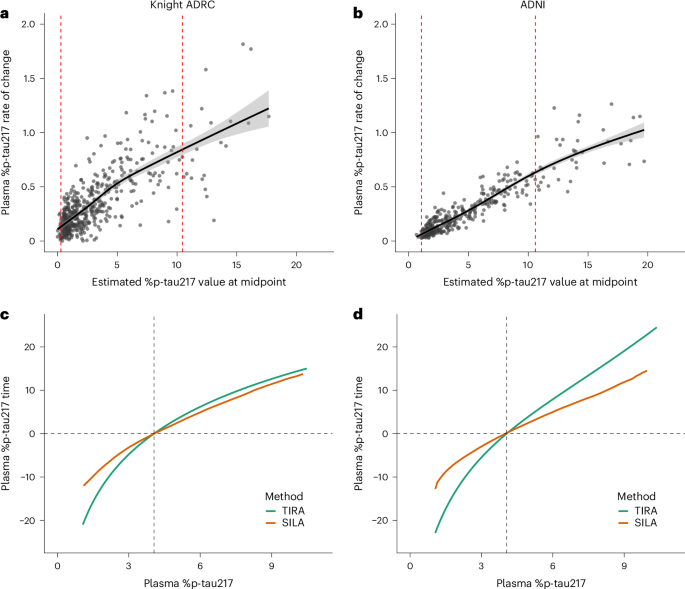
Columbus, Ohio, is currently facing a measles outbreak, with six confirmed cases among children under the age of five, according to Columbus Public Health. The outbreak underscores growing concerns about declining vaccination rates and the resurgence of preventable diseases. Public health officials are actively monitoring the situation and urging parents to ensure their children are up-to-date on their measles, mumps, and rubella (MMR) vaccinations.
The cases, reported as of February 25, 2026, include five children with no recent travel history and one child who recently traveled internationally. A concerning aspect of the outbreak is that five of the six children were unvaccinated, highlighting the vulnerability of those without protection against the highly contagious virus. Columbus Public Health is contacting individuals who may have been exposed to the five cases without travel history, while working with the Ohio Department of Health regarding the case involving international travel.
Details of the Columbus Measles Outbreak
Kelli Newman, a spokesperson for Columbus Public Health, emphasized the importance of vaccination. “The best way to stay healthy and safe is making sure your children have both recommended, age-appropriate doses of the MMR vaccine,” Newman stated. “The MMR vaccine is safe and highly effective, and it is the best way to prevent the measles.” The Centers for Disease Control and Prevention (CDC) recommends children receive their first dose of the MMR vaccine between 12 and 15 months of age, and a second dose between 4 and 6 years of age.
This outbreak follows a trend of increasing measles cases in Ohio and across the United States. In 2025, Ohio reported 45 cases of measles, including an earlier outbreak in Cuyahoga County involving three children. As of February 19, 2026, the CDC reported 982 confirmed measles cases nationwide, spanning 26 states, including Ohio, and six cases among international visitors to the U.S. NBC4i reports that nearly 150 measles cases have been reported in Franklin County since the start of 2026.
Measles: A Highly Contagious Disease
Measles is a highly contagious disease that spreads through the air when an infected person coughs or sneezes. According to Columbus Public Health, the virus can remain infectious for up to two hours in the air. Symptoms include a rash, high fever, runny nose, cough, loss of appetite, and red, watery eyes. Individuals infected with measles can spread the virus to others from four days before the rash appears through four days after. In some cases, measles can lead to more severe complications, including brain swelling and neurological damage.
Vaccination rates have been declining in recent years, raising concerns among public health experts. A 2025 study by researchers at Johns Hopkins University found that vaccination rates for measles have decreased in most U.S. Counties, falling from 93.92% pre-pandemic to 91.26%, below the 95% threshold needed for herd immunity. This decline increases the risk of outbreaks and the spread of the disease.
The United States experienced its worst measles outbreak in 2025 since the disease was declared eradicated in 2000, with a total of 90 cases reported in 2022. Prior outbreaks in Franklin County in 2025 included one involving an unvaccinated child who had traveled abroad and another linked to the New Albany-Plains Local Schools’ Early Learning Center, which resulted in a temporary closure of the school in October 2025.
Columbus Public Health encourages parents with questions or concerns about measles and vaccination to contact their healthcare provider. More information about measles can also be found on the City of Columbus Public Health website.
Health officials will continue to monitor the situation closely and provide updates as they become available. The ongoing outbreak serves as a critical reminder of the importance of vaccination in protecting individuals and communities from preventable diseases.
Please share this information with your friends and family to support raise awareness about the measles outbreak and the importance of vaccination. Your comments and questions are welcome below.
Disclaimer: This information is for general knowledge and informational purposes only, and does not constitute medical advice. It is essential to consult with a qualified healthcare professional for any health concerns or before making any decisions related to your health or treatment.