Rare Blood Disorder Shows Resistance to Common Treatment, New Research Reveals
Table of Contents
- 1. Rare Blood Disorder Shows Resistance to Common Treatment, New Research Reveals
- 2. What specific changes in Absolute lymphocyte Count (ALC) would most strongly indicate venetoclax is *not* effectively treating CLL?
- 3. Decoding Hematologic Biomarkers: How Blood Cell Patterns Signal Ineffective Venetoclax Treatment
- 4. Understanding Venetoclax and its Target: BCL-2
- 5. The Role of Hematologic Biomarkers in Venetoclax Response
- 6. Specific Blood Cell Patterns Indicating ineffective Treatment
- 7. 1. Plateauing or Rising ALC in CLL
- 8. 2. Insufficient Blast Reduction in AML
- 9. 3. Prolonged and Severe Cytopenias
- 10. 4. Atypical Lymphocytosis
- 11. Benefits of Proactive Biomarker Monitoring
- 12. Practical Tips for Hematologic Biomarker Interpretation
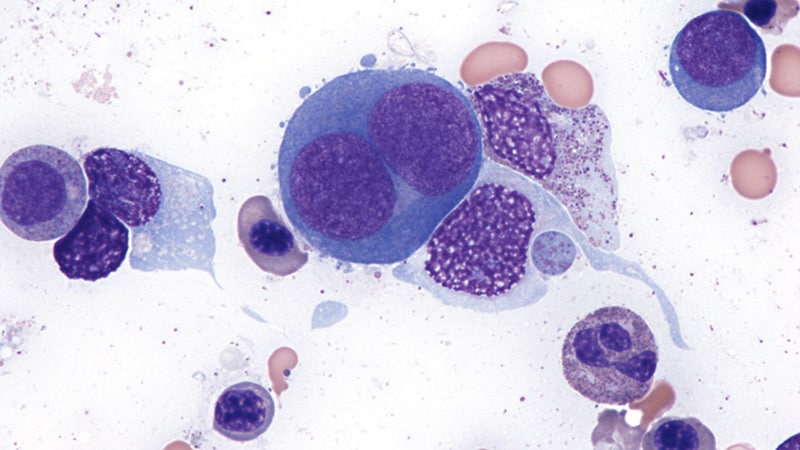
HOUSTON, TX – A distinct subtype of a rare blood cancer, erythroid-predominant myelodysplastic neoplasms (EP MDS), exhibits unique genetic characteristics and a surprising resistance to a commonly used therapy, according to a new study from The University of Texas MD Anderson Cancer Center. The findings, published in Leukemia, suggest a need for choice treatment strategies for patients with this aggressive form of the disease.
MDS is a group of disorders were the bone marrow doesn’t produce enough healthy blood cells. EP MDS, accounting for roughly 18% of all MDS cases, is characterized by a high proportion of developing red blood cells in the bone marrow. Researchers analyzed data from 371 newly diagnosed MDS patients and a separate cohort of 112 patients receiving treatment with a hypomethylating agent plus venetoclax.
The study revealed that EP MDS patients frequently harbor mutations in the TP53 gene – a critical tumor suppressor – as well as mutations in BCOR and WT1. Importantly, the research identified three genetic subgroups within EP MDS, each with significantly different survival rates: TP53 mutant (median survival 11.4 months),splicing mutant (survival not yet reached),and a third,less defined group (19.5 months).
However, the most striking finding concerned the effectiveness of venetoclax, a drug often used to treat MDS. Patients with EP MDS treated with venetoclax experienced significantly higher rates of conversion to acute leukemia (32% vs. 12%) and markedly worse overall survival (8.3 months vs. not reached) compared to those without the EP subtype.
Further investigation revealed the reason for this resistance. EP MDS cells appear to rely more heavily on the BCL-XL protein for survival, rather than BCL2 – the protein targeted by venetoclax. Immunohistochemical analysis showed significantly higher levels of BCL-XL in EP MDS cells compared to other MDS subtypes.
“Our findings provide a detailed characterization of EP MDS and demonstrate its distinct molecular and clinical profile,” said lead author Dr. Alexandre Bazinet. “These patients experience poor outcomes with currently available therapies,supporting the growth of alternative therapeutic strategies,especially BCL-XL inhibitors.”
The researchers acknowledge the study’s limitations, including being conducted at a single center and the relatively small number of EP MDS cases, which limited the ability to fully define genetic subgroups. they also emphasize the need for further research to confirm the link between increased BCL-XL expression and treatment resistance, and to determine whether TP53 mutations or the erythroid-differentiation bias are primarily responsible for the poor response to venetoclax.
This research underscores the importance of precise diagnosis and personalized treatment approaches in MDS, paving the way for future clinical trials evaluating novel therapies tailored to the unique characteristics of EP MDS.
https://www.nature.com/articles/s41375-025-02711-6
What specific changes in Absolute lymphocyte Count (ALC) would most strongly indicate venetoclax is *not* effectively treating CLL?
Decoding Hematologic Biomarkers: How Blood Cell Patterns Signal Ineffective Venetoclax Treatment
Understanding Venetoclax and its Target: BCL-2
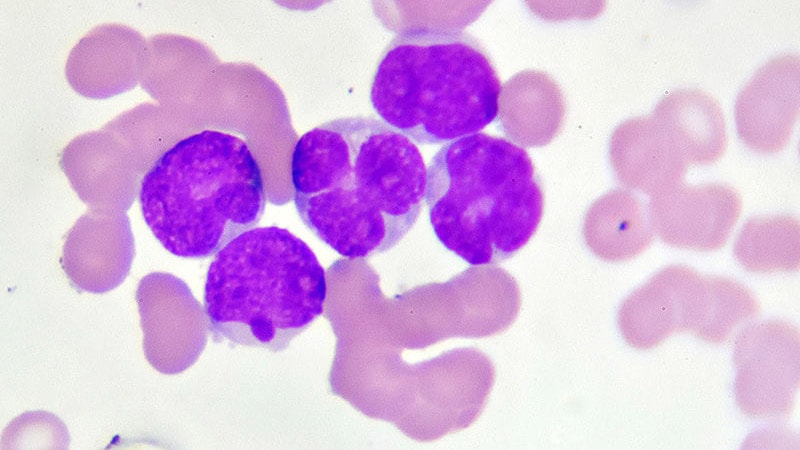
Venetoclax, a BCL-2 inhibitor, has revolutionized the treatment landscape for chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). Its mechanism centers on inducing apoptosis – programmed cell death – in cancer cells by neutralizing the anti-apoptotic protein BCL-2. However, not all patients respond optimally. Monitoring hematologic biomarkers is crucial for early detection of treatment failure and guiding clinical decisions. Recognizing patterns in blood cell counts can provide vital clues about venetoclax resistance or inadequate response. This article delves into the specific blood cell changes that signal potential issues with venetoclax therapy, focusing on practical interpretation for hematologists and oncologists.
The Role of Hematologic Biomarkers in Venetoclax Response
Hematologic biomarkers, readily available through routine complete blood counts (CBC) with differential, offer a non-invasive window into the drug’s efficacy. These aren’t just numbers; they reflect the dynamic interplay between venetoclax, the leukemia cells, and the patient’s bone marrow.Key biomarkers to monitor include:
Absolute Lymphocyte Count (ALC): In CLL, a meaningful and sustained decrease in ALC is a hallmark of effective venetoclax treatment.
Blast Percentage: For AML, a reduction in blast percentage (immature blood cells) is the primary indicator of response.
Platelet Count: Often suppressed during initial venetoclax treatment due to on-target, off-tumor effects, platelet recovery is essential for continued therapy.
Neutrophil Count: Similar to platelets, neutrophil counts may initially decrease but should stabilize or recover with treatment.
Red Blood Cell (RBC) Count & Hemoglobin: Monitoring for anemia, which can be exacerbated by venetoclax or underlying disease.
Specific Blood Cell Patterns Indicating ineffective Treatment
1. Plateauing or Rising ALC in CLL
A persistent plateau or, worse, a rise in ALC despite several cycles of venetoclax strongly suggests treatment failure. This could be due to:
BCL-2 Mutation: Mutations within the BCL-2 gene can confer resistance to venetoclax.
TP53 Aberrations: TP53 mutations are associated with poorer outcomes and reduced venetoclax sensitivity.
Option Survival Pathways: Leukemia cells may upregulate other anti-apoptotic pathways, bypassing BCL-2 inhibition.
Actionable Step: Consider BCL-2 and TP53 mutation analysis. Explore combination therapies targeting alternative survival pathways.
2. Insufficient Blast Reduction in AML
In AML, a less than 50% reduction in bone marrow blasts after 1-2 cycles of venetoclax combined with azacitidine or decitabine is considered suboptimal response. This warrants investigation into:
IDH1/2 Mutations: The presence of IDH1/2 mutations can influence venetoclax response.
FLT3-ITD: FLT3-ITD mutations are often associated with resistance.
Mitochondrial Fitness: Leukemia cells with higher mitochondrial fitness may be less reliant on BCL-2 for survival.
Actionable Step: Perform comprehensive genomic profiling to identify resistance mechanisms. Consider alternative AML therapies or clinical trials.
3. Prolonged and Severe Cytopenias
While initial cytopenias (low blood cell counts) are expected, prolonged or worsening thrombocytopenia (low platelets) and neutropenia (low neutrophils) can signal issues.
Bone Marrow Suppression: Venetoclax can directly suppress bone marrow function.
Secondary Infections: Neutropenia increases the risk of life-threatening infections.
Drug-Drug Interactions: Interactions with other medications can exacerbate cytopenias.
Actionable Step: Implement prophylactic antibiotics and growth factors (e.g., G-CSF for neutropenia). Review the patient’s medication list for potential interactions. Consider dose reduction or interruption of venetoclax.
4. Atypical Lymphocytosis
In some CLL cases, venetoclax can induce an atypical lymphocytosis characterized by a transient increase in atypical lymphocytes. This is usually a sign of effective cell death, but a sustained atypical lymphocytosis without a corresponding decrease in ALC can be concerning.
Actionable Step: Monitor closely and consider bone marrow evaluation to rule out other causes.
Benefits of Proactive Biomarker Monitoring
Early Intervention: Identifying ineffective treatment early allows for timely adjustments to the treatment plan.
Personalized Therapy: Genomic profiling guided by biomarker patterns enables personalized treatment strategies.
Improved Outcomes: Proactive monitoring can improve response rates and overall survival.
Reduced Toxicity: Avoiding continued treatment with a failing regimen minimizes unnecessary toxicity.
Practical Tips for Hematologic Biomarker Interpretation
Establish Baseline: Obtain a comprehensive CBC with differential before initiating venetoclax.
Regular Monitoring: Monitor CBC at least weekly during the frist cycle, then bi-weekly or monthly thereafter.
Trend Analysis: Focus on trends in biomarker values rather than isolated numbers.
Correlation with Clinical Symptoms: Integrate biomarker data with the patient’s clinical presentation.
* collaboration: Collaborate with a hematopath