Hope Emerges for Cancer Patients with Novel Pathway Inhibitor
Table of Contents
- 1. Hope Emerges for Cancer Patients with Novel Pathway Inhibitor
- 2. Understanding the Hippo-YAP-TEAD pathway
- 3. Clinical trial Highlights and Mesothelioma Response
- 4. Key Trial Facts
- 5. The Future of Cancer Treatment
- 6. Understanding Cancer Pathways
- 7. Frequently Asked Questions about Hippo-YAP-TEAD Inhibitors
- 8. What specific patient populations might benefit most from VT3989 based on the tumor types included in the Phase 1/2 clinical trial?
- 9. YAP/TEAD Inhibitor VT3989 for Solid Tumors: A Phase 1/2 Clinical Trial
- 10. Understanding the YAP/TEAD Pathway & Cancer
- 11. VT3989: A First-in-Class YAP/TEAD Inhibitor
- 12. mechanism of Action: How VT3989 Works
- 13. Phase 1/2 Clinical Trial: Key Findings & data
- 14. Patient Selection & Biomarker Importance
- 15. Future Directions & Clinical development
- 16. Real-World Implications & Patient Advocacy
A groundbreaking treatment approach is offering new optimism in the fight against advanced cancers. Researchers have announced encouraging early results from clinical trials involving a first-in-class inhibitor targeting the Hippo-YAP-TEAD pathway. The findings, presented at the European society for Medical Oncology Congress 2025, suggest the therapy is safe and shows potential for meaningful clinical benefit, especially for individuals battling mesothelioma.
Understanding the Hippo-YAP-TEAD pathway
The hippo-YAP-TEAD pathway is a crucial signaling cascade regulating organ size and tissue repair.However, in many cancers, this pathway becomes abnormally activated, driving uncontrolled cell growth and tumor progression. Blocking this pathway has become a key target for innovative cancer therapies. This new inhibitor represents a significant step forward in achieving that goal.
Clinical trial Highlights and Mesothelioma Response
The clinical trials focused on patients with locally advanced or metastatic solid tumors, encompassing a range of cancer types, but with a specific emphasis on mesothelioma-a rare and aggressive cancer affecting the lining of the lungs, abdomen, or heart. Initial data indicates the inhibitor was well-tolerated by patients, with no unacceptable levels of toxicity reported. More importantly, the trials demonstrated encouraging response rates among mesothelioma patients.
According to the National Cancer Institute, approximately 2,500 Americans are diagnosed with mesothelioma each year. National Cancer Institute. The prognosis for mesothelioma patients remains poor, underscoring the urgent need for new and effective treatments.
Key Trial Facts
| Trial Focus | Pathway Inhibited | Cancer Types | Key Finding |
|---|---|---|---|
| Advanced Solid Tumors | Hippo-YAP-TEAD | Mesothelioma & others | Safe & Promising Response |
Did You Know? The Hippo pathway was first identified in fruit flies, where it controls organ size during progress.
The Future of Cancer Treatment
While these results are preliminary, they represent a significant stride in precision oncology. The development of targeted therapies, such as this Hippo-YAP-TEAD inhibitor, is shifting cancer treatment away from broad-spectrum chemotherapy towards more tailored approaches. The goal is to disrupt the specific molecular drivers of each patient’s cancer,minimizing side effects and maximizing efficacy.
Pro Tip: Staying informed about clinical trials and advances in cancer research can empower patients and their families to make more informed decisions about their care.
Further research and larger-scale clinical trials are now planned to confirm these findings and explore the full potential of this innovative therapy. Researchers are optimistic that this inhibitor could become a valuable addition to the cancer treatment arsenal.
Understanding Cancer Pathways
Cancer pathways are complex networks of signaling molecules within cells.These pathways control essential cellular processes like growth, division, and survival.When these pathways are disrupted, it can lead to uncontrolled cell growth and the development of cancer. Targeting these pathways with drugs like the Hippo-YAP-TEAD inhibitor offers a promising way to halt cancer progression.
Frequently Asked Questions about Hippo-YAP-TEAD Inhibitors
-
What is a Hippo-YAP-TEAD inhibitor?
It is a type of drug that blocks the Hippo-YAP-TEAD signaling pathway, which is often overactive in cancer cells and drives tumor growth.
-
What types of cancer could benefit from this treatment?
Early trials have shown promise in mesothelioma, but research is ongoing to explore its effectiveness in other solid tumors.
-
Is this treatment currently available to patients?
No, it is indeed still in clinical trials and is not yet approved for widespread use.
-
What are the potential side effects of this inhibitor?
Initial data suggests the treatment is well-tolerated, but further research is needed to fully understand its side effect profile.
-
How does the Hippo-YAP-TEAD pathway contribute to cancer development?
When this pathway is overactive, it promotes uncontrolled cell growth, inhibits cell death, and contributes to the spread of cancer.
What are your thoughts on the potential of pathway inhibitors in cancer treatment? Share your comments below, and let’s continue the conversation.
What specific patient populations might benefit most from VT3989 based on the tumor types included in the Phase 1/2 clinical trial?
YAP/TEAD Inhibitor VT3989 for Solid Tumors: A Phase 1/2 Clinical Trial
Understanding the YAP/TEAD Pathway & Cancer
The Yes-associated protein (YAP) adn transcriptional co-activator with PDZ-binding motif (TEAD) pathway is a crucial signaling cascade involved in organ size control and tissue homeostasis. Aberrant activation of this pathway is frequently observed in a wide range of solid tumors, including those of the breast, lung, liver, and pancreas. This dysregulation promotes uncontrolled cell growth, tumor progression, and resistance to conventional therapies. Targeting YAP/TEAD,therefore,represents a promising new avenue in cancer treatment. YAP-TEAD inhibition is gaining traction as a potential therapeutic strategy.
VT3989: A First-in-Class YAP/TEAD Inhibitor
VT3989 is an orally bioavailable, small-molecule inhibitor specifically designed to disrupt the interaction between YAP and TEAD proteins. Unlike many cancer therapies that target the cancer cells directly,VT3989 aims to modulate the signaling pathways driving cancer cell proliferation. This approach offers the potential for broader efficacy and reduced progress of resistance. It’s considered a novel cancer therapy and a critically important step forward in precision oncology.
mechanism of Action: How VT3989 Works
VT3989 functions by binding to TEAD proteins, preventing them from interacting with YAP.This disruption effectively silences the downstream signaling cascade, leading to:
* Reduced expression of target genes involved in cell proliferation and survival.
* Inhibition of tumor growth.
* Increased sensitivity to other cancer treatments, such as chemotherapy and immunotherapy.
* Potential for metastasis prevention by reducing epithelial-mesenchymal transition (EMT).
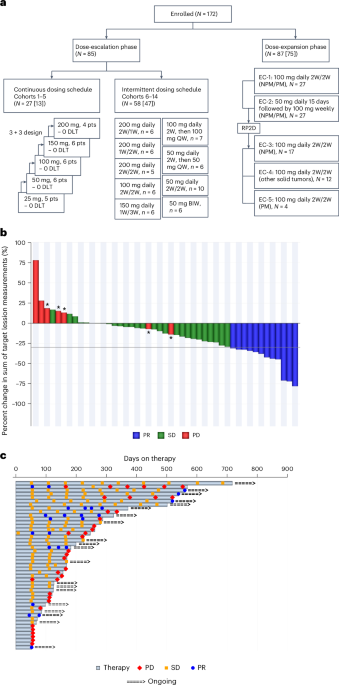
Phase 1/2 Clinical Trial: Key Findings & data
The ongoing phase 1/2 clinical trial evaluating VT3989 in patients with advanced solid malignancies has yielded encouraging preliminary results.The trial, designed to assess safety, tolerability, and preliminary efficacy, enrolled patients with various tumor types, including:
* Non-Small Cell Lung cancer (NSCLC)
* Pancreatic Cancer
* Breast Cancer (specifically, hormone receptor-positive, HER2-negative)
* Cholangiocarcinoma
Key observations from the trial (as of October 2025):
- Safety & Tolerability: VT3989 demonstrated a manageable safety profile, with the most common adverse events being fatigue, nausea, and diarrhea. These were generally mild to moderate in severity and responsive to supportive care. No dose-limiting toxicities were reported in the initial dose-escalation phase.
- Pharmacokinetics: The drug exhibited favorable pharmacokinetic properties, with good oral bioavailability and predictable drug exposure.
- Preliminary Efficacy: While the trial is not powered for definitive efficacy assessment at this stage, several patients experienced stable disease or partial responses, especially in those with tumors harboring specific genetic alterations known to activate the YAP/TEAD pathway.
- biomarker Analysis: Researchers are actively analyzing tumor biopsies to identify biomarkers predictive of response to VT3989. Initial data suggests that tumors with high YAP/TEAD pathway activity are more likely to benefit from treatment. Predictive biomarkers are crucial for patient selection.
Patient Selection & Biomarker Importance
Identifying patients most likely to respond to VT3989 is paramount. Current research focuses on:
* YAP/TEAD Target Gene Expression: Assessing the expression levels of genes regulated by the YAP/TEAD pathway in tumor samples.
* Genetic Mutations: Identifying mutations in genes that activate YAP/TEAD signaling,such as FGFR3 and PDGFRA.
* Protein Expression: Measuring YAP and TEAD protein levels in tumor tissue.
* Liquid Biopsies: Utilizing circulating tumor DNA (ctDNA) to detect YAP/TEAD pathway alterations.
Personalized medicine approaches, guided by biomarker analysis, will likely be essential for maximizing the benefit of VT3989.
Future Directions & Clinical development
The Phase 1/2 trial is continuing to enroll patients, and further data analysis is expected to refine our understanding of VT3989’s efficacy and safety. Future clinical development plans include:
* Phase 2 Expansion Cohorts: Expanding the trial to include larger cohorts of patients with specific tumor types demonstrating promising activity.
* Combination Therapy studies: Evaluating VT3989 in combination with other cancer treatments, such as chemotherapy, immunotherapy, and targeted therapies. Drug combinations may enhance efficacy.
* Phase 3 Randomized Controlled Trials: Conducting large-scale, randomized controlled trials to confirm the efficacy of VT3989 and establish it as a standard of care for select cancer patients.
Real-World Implications & Patient Advocacy
The development of VT3989 represents a significant advancement in the field of targeted cancer therapy. While still in the early stages of clinical development, this first-in-class YAP/TEAD inhibitor offers hope for patients