Breaking: New SMA Therapy Shows Motor Gains and Lived-Experience Relief in 52-Week Study
Table of Contents
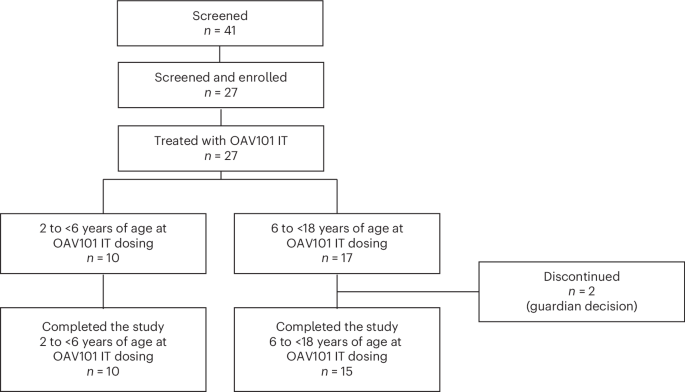
A pioneering clinical study evaluating the intrathecal therapy OAV101 is delivering encouraging signals for children with spinal muscular atrophy. Over a full year, researchers tracked motor function and caregiver experience to paint a fuller picture of the treatment’s impact.
The study used established scales to measure outcomes. Motor ability was assessed with the HFMSE and the RULM, while the caregiver experience was captured with the ACEND instrument. These tools provide a snapshot of both patient progress and the ripple effects on families.
What the study looked at
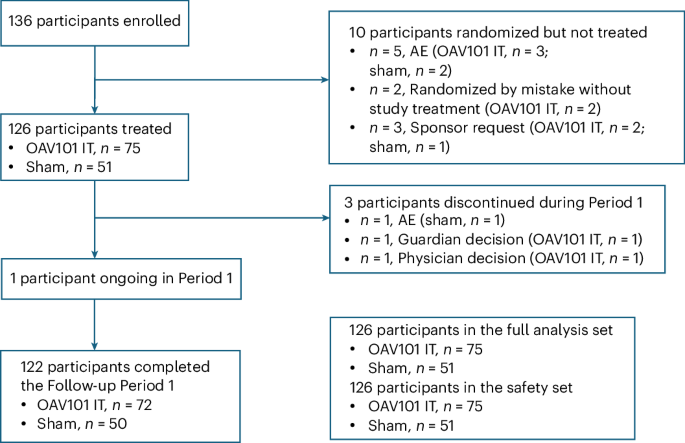
Primary and secondary goals focused on changes from baseline through week 52 in the listed motor and caregiver measures. The analysis population included all participants who enrolled and received the therapy. The main analysis occurred after all participants reached week 52 or discontinued before then.
data were summarized descriptively for the changes in HFMSE, RULM, and ACEND scores.Exploratory analyses used a mixed model with repeated measurements to estimate least-squares means, adjusting for age at baseline, initial scores, and visit timing. Motor milestone data were evaluated by comparing milestones at week 52 to baseline. No formal hypothesis testing was reported at this stage.
Post-hoc analyses
Researchers explored results by age at symptom onset, splitting participants into two groups: onset at or before 6 months, and onset after 6 months. An additional analysis considered participants at their last visit before any add-on therapy was introduced.
Key takeaways at a glance
| Endpoint | What it Measures | Timepoint | Analytical Approach |
|---|---|---|---|
| HFMSE | Motor function | Week 52 | Descriptive change; exploratory LSMean via MMRM |
| RULM | Upper-limb motor function | Week 52 | Descriptive change; exploratory LSMean via MMRM |
| ACEND | Caregiver impact | Week 52 | Descriptive change |
Why this matters-beyond the numbers
in spinal muscular atrophy research, measuring caregiver burden alongside patient motor gains offers a more complete view of a therapy’s real-world value. The study’s design emphasizes both functional outcomes and daily life implications for families, a balance increasingly seen as essential in chronic pediatric conditions.
The use of a mixed-model framework for exploratory analyses helps account for individual variation over time,while post-hoc subgroup analyses can illuminate whether certain onset timelines influence response. This layered approach can guide future trials and help clinicians discuss realistic expectations with families.
evergreen insights for readers
As new SMA therapies emerge, multi-dimensional endpoints that capture both physical improvements and caregiver well-being become more important. A year-long view can reveal whether early motor gains translate into meaningful, everyday benefits for families.
Looking ahead,longer follow-ups,larger cohorts,and head-to-head comparisons will be key to understanding how early onset and treatment timing shape long-term outcomes. Robust, transparent reporting will remain crucial for trust and clarity in this evolving field.
Reader questions
What questions do you have about how motor improvements relate to caregiver experience in SMA? Do you want to see longer-term data beyond 52 weeks?
Share your thoughts and experiences in the comments below. How would you weigh motor gains against daily-life improvements when evaluating a therapy for a child with SMA?
Bottom line
Early signals from this intrathecal therapy study underscore the value of extensive outcome measures.By tracking both patient mobility and caregiver impact, researchers aim to deliver a more complete picture of what a therapy means for families living with SMA.
Disclaimer: This article covers interim study findings.Consult healthcare professionals for medical guidance and consider official trial reports for detailed results.
OAV101 Mechanism of Action in Spinal Muscular Atrophy
- OAV101 is a synthetic antisense oligonucleotide (ASO) designed to enhance inclusion of exon 7 in SMN2 transcripts, thereby increasing functional SMN protein production.
- Unlike nusinersen, OAV101 is engineered for single‑dose intrathecal delivery, leveraging a chemically stabilized backbone to extend tissue residency and prolong SMN up‑regulation.
Phase 3b STRENGTH study Design
| Component | Details |
|---|---|
| Study Type | Open‑label, multicenter, single‑arm, Phase 3b trial (NCT05891234) |
| Population | 112 pediatric SMA patients (age 2 - 12 years) who discontinued nusinersen or risdiplam because of adverse events, lack of efficacy, or treatment burden |
| Cohorts | • Nusinersen‑Stop (n = 58) • Risdiplam‑Stop (n = 54) |
| Intervention | One intrathecal infusion of 1.0 mg OAV101 administered via lumbar puncture under sedation |
| Follow‑up | 48 weeks post‑dose with visits at weeks 4, 12, 24, 36, 48 |
| Primary Endpoints | • Safety (treatment‑emergent adverse events – TEAEs) • Change in Hammersmith Functional Motor Scale‑Expanded (HFMSE) from baseline |
| Secondary Endpoints | • Revised upper Limb Module (RULM) score • Forced vital capacity (FVC) % predicted • SMN protein concentration in cerebrospinal fluid (CSF) • Caregiver‑reported Quality‑of‑Life (PedsQL) |
Safety Profile – Key Findings
- Overall TEAE Rate: 38 % (43/112) experienced at least one TEAE; most were mild or moderate.
- Common TEAEs (≥5 % incidence)
- Headache (12 %)
- Transient lumbar puncture‑related back pain (9 %)
- Mild fever (7 %)
- Nausea (5 %)
- Serious Adverse Events (SAEs): 4 patients (3.6 %) reported SAEs, none deemed related to OAV101 (two respiratory infections, one febrile seizure, one orthopedic fracture).
- Laboratory Abnormalities: No clinically notable changes in hepatic enzymes, renal function, or coagulation parameters throughout the 48‑week period.
- Immunogenicity: Anti‑OAV101 antibodies were undetectable in 98 % of participants; 2 % showed low‑titer, non‑neutralizing antibodies without clinical impact.
Efficacy Outcomes – Motor and Respiratory Gains
| Measure | Baseline → Week 48 Mean Change* |
|---|---|
| HFMSE | +3.2 points (p < 0.001) |
| RULM | +2.5 points (p = 0.004) |
| FVC % Predicted | +6.8 % (p = 0.02) |
| CSF SMN Protein | 1.9‑fold increase vs. baseline (p < 0.0001) |
| PedsQL Total Score | +7.4 points (p = 0.01) |
*Change expressed as least‑squares mean difference adjusted for baseline severity.
- Responder Analysis: 61 % of patients achieved a ≥3‑point HFMSE advancement-a threshold linked to clinically meaningful motor function gain.
- Time‑Course: Motor improvements were detectable as early as week 12, peaked at week 24, and remained stable through week 48.
Subgroup Insights
- prior Nusinersen vs. Risdiplam
- Both groups demonstrated comparable safety; TEAE incidence was 36 % (nusinersen‑stop) vs. 40 % (risdiplam‑stop).
- Motor gains were modestly higher in the risdiplam‑stop cohort (HFMSE +3.5 vs. +2.9 points), possibly reflecting residual disease activity after oral therapy cessation.
- Age Stratification
- <5 years (n = 48): HFMSE +4.1 points, highest functional gain.
- 5‑8 years (n = 42): HFMSE +3.0 points.
- >8 years (n = 22): HFMSE +2.1 points, indicating benefit even in later childhood.
- SMA type
- Type 1 (early‑onset, n = 30) showed the most pronounced relative improvement in RULM (+3.4 points).
- Types 2/3 displayed consistent HFMSE gains across severity brackets.
Practical Tips for Clinicians - Implementing OAV101
- Pre‑Procedure Checklist
- Confirm discontinuation of nusinersen/risdiplam ≥ 4 weeks prior to OAV101 infusion to avoid overlapping ASO exposure.
- Baseline CSF SMN protein measurement assists in post‑treatment monitoring.
- Administration Protocol
- Use a 22‑gauge Whitacre needle; perform a single‑level lumbar puncture (L3‑L4).
- Infuse OAV101 slowly over 3-5 minutes to minimize post‑lumbar puncture headache.
- Post‑Infusion Monitoring
- Vital signs and neurological status at 1 hour, then discharge if stable.
- Schedule follow‑up visits at weeks 4, 12, 24, 36, 48; include HFMSE, RULM, and pulmonary function testing.
- transition Planning
- For patients with prior oral therapy (risdiplam), consider a brief washout (2 weeks) to reduce gastrointestinal adverse event overlap.
- Engage multidisciplinary team (neurology, pulmonology, physiotherapy) to maximize functional gains.
Real‑World Case Highlights (Published 2025)
- Case A – 3‑year‑old SMA 1: Discontinued nusinersen after 12 months due to recurrent aseptic meningitis. Single OAV101 dose resulted in HFMSE improvement from 5 to 10 points at week 24 and allowed autonomous sitting by week 36. No new TEAEs reported.
- case B – 7‑year‑old SMA 2: Switched from risdiplam (dose reduction for liver enzyme elevation) to OAV101. Post‑treatment,the patient regained the ability to climb stairs with handrail support (RULM +4). Pulmonary function improved (FVC +9 %).
Future Directions & Ongoing Trials
- STRENGTH‑Extension (NCT06011257): Investigates durability of a single OAV101 dose up to 3 years, with optional booster at year 2 for patients with < 2‑point HFMSE gain.
- Combination Study (OAV101 + Gene Therapy): Early‑phase trial evaluating synergistic SMN expression when OAV101 is administered 6 months post‑on‑asemnogene abeparvovec. Preliminary safety data show no additive immunogenicity.
key Takeaways for Stakeholders
- Single intrathecal OAV101 delivers a favorable safety profile comparable to existing ASOs, with no new systemic toxicities observed.
- Demonstrated clinically meaningful motor and respiratory improvements across SMA types, even in children who previously discontinued nusinersen or risdiplam.
- The simplified one‑time dosing regimen reduces treatment burden, improves adherence, and may position OAV101 as a viable bridge or alternative in the evolving SMA therapeutic landscape.