Beyond Ozempic: How Brain Cell Signaling Could Unlock the Next Generation of Weight-Loss Drugs
For millions grappling with obesity, medications like Ozempic and Wegovy have offered a beacon of hope, delivering unprecedented weight loss results. But why do these drugs, and particularly the newer co-agonists like Zepbound (tirzepatide), work so effectively? A groundbreaking new study published in Cell Metabolism reveals a surprising piece of the puzzle: it’s not just about what happens in the gut, but also about a previously overlooked conversation happening within the brain, specifically involving specialized brain cells called oligodendrocytes. This discovery isn’t just academic; it could revolutionize how we approach obesity treatment, paving the way for more targeted therapies and personalized dosing strategies.
The Brain’s Hidden Role in Weight Loss
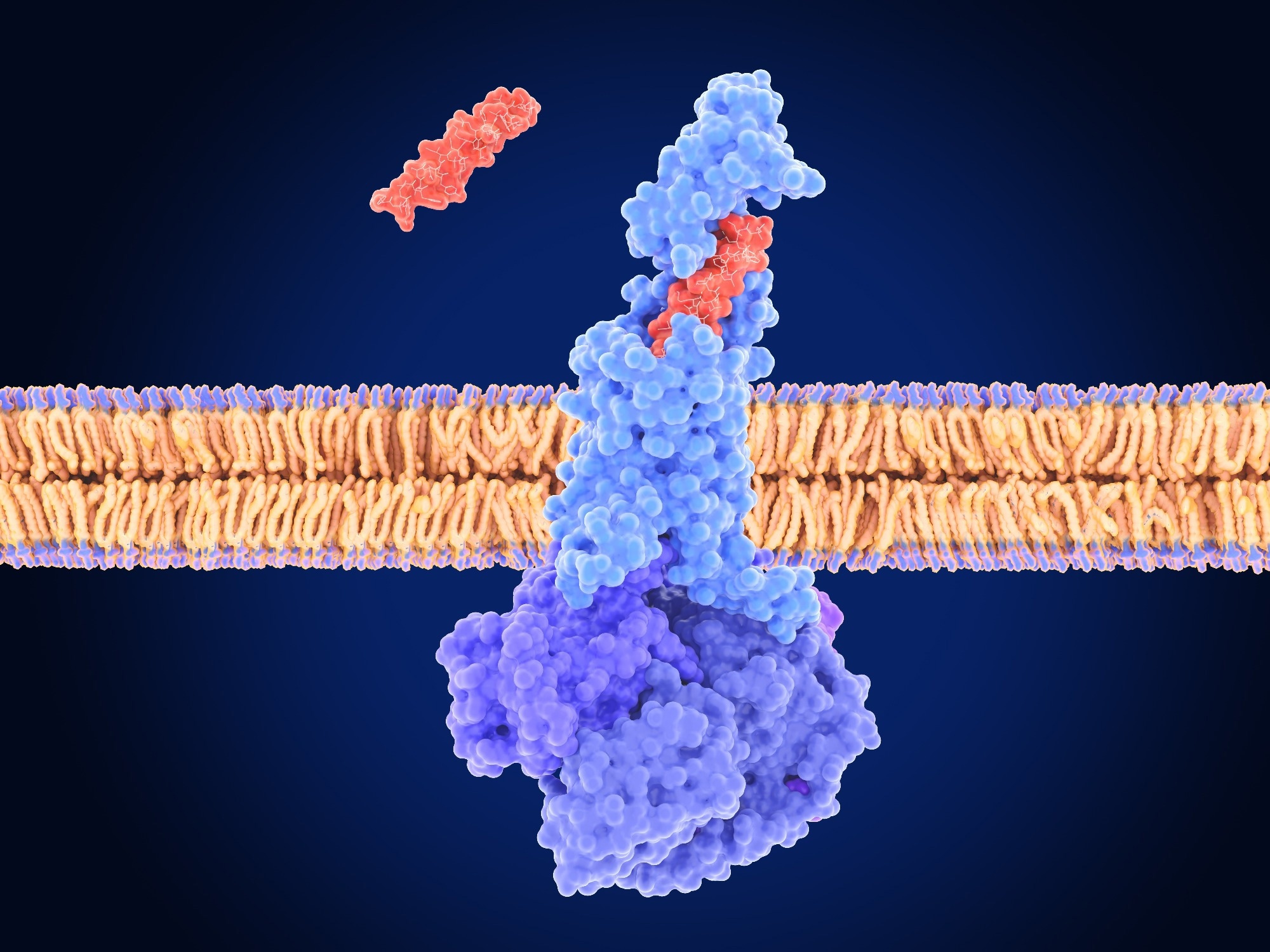
Traditionally, GLP-1 receptor agonists (GLP-1RAs) and GIPR agonists were understood to work primarily by mimicking natural hormones that regulate appetite and insulin secretion. However, recent research, and now this study, points to a more complex picture. The key lies in the blood-brain barrier (BBB), a protective shield that tightly controls what enters the brain. Getting these drugs *into* the brain appears crucial for maximizing their effects, and this is where oligodendrocytes come into play.
Oligodendrocytes, long known for their role in forming the myelin sheath that insulates nerve fibers, are now revealed to be active gatekeepers at a critical brain region called the median eminence (ME). The ME acts as a bridge between the bloodstream and the hypothalamus, the brain’s appetite control center. Researchers discovered that activating GIPR signaling in these oligodendrocytes increases the permeability of the ME, essentially opening the door for GLP-1R agonists to reach their targets more effectively.
“This study elegantly demonstrates that oligodendrocytes aren’t just passive support cells in the brain, but active participants in regulating metabolic pathways,” says Dr. Anya Sharma, a neuroendocrinologist at the National Institutes of Health (NIH). “Understanding this mechanism could explain why co-agonists, targeting both GIP and GLP-1 receptors, show superior efficacy compared to single-agonist therapies.”
Unlocking the Mechanism: Myelin, Vascular Permeability, and AVP Neurons
The research team used sophisticated techniques, including light-sheet microscopy and genetic manipulation in mice, to unravel the intricate details of this process. They found that activating GIPR in oligodendrocytes led to increased production of vascular endothelial growth factor A (VEGF-A), a protein that promotes blood vessel growth and permeability. This, in turn, resulted in denser networks of capillaries within the ME, allowing more of the GLP-1R agonist to cross into the brain.
But the story doesn’t end there. The study also revealed a surprising pathway: GLP-1R agonists don’t just diffuse freely into the brain. They actually hitch a ride along myelinated axon bundles, specifically those belonging to arginine vasopressin (AVP) neurons. These neurons play a critical role in regulating appetite and energy expenditure. By bypassing the BBB via these myelinated pathways, the drugs can directly influence AVP neuron activity, amplifying their weight-loss effects.
GLP-1R agonists are now understood to access the brain via a novel mechanism, utilizing myelinated axons in the median eminence.
What Does This Mean for Zepbound (Tirzepatide)?
Zepbound, a dual GIP/GLP-1 receptor agonist, consistently demonstrates greater weight loss than drugs like Ozempic (semaglutide), which primarily targets the GLP-1 receptor. This new research provides a compelling explanation. By simultaneously activating both GIPR and GLP-1R, Zepbound appears to maximize the oligodendrocyte-mediated pathway, enhancing brain access and boosting the drug’s overall efficacy. The synergistic effect isn’t just about hitting two targets; it’s about unlocking a more efficient delivery system to the brain.
Future Trends and Implications
This discovery opens up several exciting avenues for future research and therapeutic development:
- Biomarker Development: VEGF-A levels in the blood could potentially serve as a biomarker to predict an individual’s response to GLP-1R agonists. Higher baseline VEGF-A might indicate greater potential for brain drug delivery and, therefore, a more robust weight-loss response.
- Targeted Therapies: Researchers could explore strategies to directly enhance GIPR signaling in oligodendrocytes, potentially boosting the efficacy of existing GLP-1R agonists or developing entirely new therapies.
- Personalized Dosing: Imaging techniques to assess ME access could help clinicians tailor drug dosages to individual patients, optimizing treatment outcomes and minimizing side effects.
- Beyond Obesity: The role of oligodendrocytes and the ME in regulating brain access could extend to other neurological disorders, potentially opening up new treatment avenues for conditions like Alzheimer’s disease and Parkinson’s disease.
Key Takeaway: The brain is not a passive bystander in weight loss. Understanding the intricate interplay between peripheral hormones, brain cells, and the blood-brain barrier is crucial for developing more effective and personalized obesity treatments.
Did you know? Oligodendrocytes make up approximately 50% of the cells in the central nervous system, highlighting their critical role in brain function beyond just myelin production.
The Road Ahead: Challenges and Opportunities
While this study represents a significant leap forward, several challenges remain. The research was conducted in mice, and further studies are needed to confirm these findings in humans. Additionally, the OL Gipr knockout model achieved only partial deletion, and the experiments primarily focused on liraglutide. Exploring the effects of other GLP-1R agonists, including semaglutide and tirzepatide, is crucial.
However, the potential benefits are immense. By unraveling the brain’s role in weight loss, we’re moving closer to a future where obesity treatment is not just about suppressing appetite, but about restoring the brain’s natural regulatory mechanisms. This could lead to more sustainable weight loss, improved metabolic health, and a better quality of life for millions.
Frequently Asked Questions
Q: Will this research lead to new weight-loss drugs?
A: While it’s too early to say definitively, this research identifies a novel target – oligodendrocyte GIPR signaling – that could be exploited to develop more effective weight-loss therapies. It’s a promising area for future drug development.
Q: Could this explain why some people respond better to GLP-1 drugs than others?
A: Potentially. Variations in GIPR expression in oligodendrocytes or differences in ME vascular permeability could contribute to individual differences in drug response. Further research is needed to investigate this.
Q: Is there anything I can do now to improve my response to GLP-1 medications?
A: Maintaining a healthy lifestyle, including a balanced diet and regular exercise, is always recommended. Discuss any concerns or questions you have with your healthcare provider.
What are your thoughts on the future of obesity treatment? Share your insights in the comments below!
Learn more about improving your metabolic health.
Stay informed about new developments in weight-loss medications.
Read the original research article in Cell Metabolism.