:
Cystitis Linked to Increased Cancer Risk, swedish Study Finds
Table of Contents
- 1. Cystitis Linked to Increased Cancer Risk, swedish Study Finds
- 2. Okay,here’s a breakdown of the key information from the provided text,organized for clarity and potential use in answering questions or summarizing the content.
- 3. Recent Study Links Urinary Tract Infections to increased risk of Urogenital Cancers
- 4. The Emerging Connection: UTIs and Cancer Risk
- 5. Understanding the Inflammatory Pathway
- 6. Specific Cancers and UTI Correlation
- 7. Identifying Risk Factors & Populations
- 8. Diagnostic Approaches & Early Detection
- 9. Prevention Strategies: Reducing UTI Risk
- 10. Case Study: Recurrent UTIs Leading to Early Bladder Cancer Detection
- 11. The Role of antibiotic Stewardship
- 12. Future Research Directions
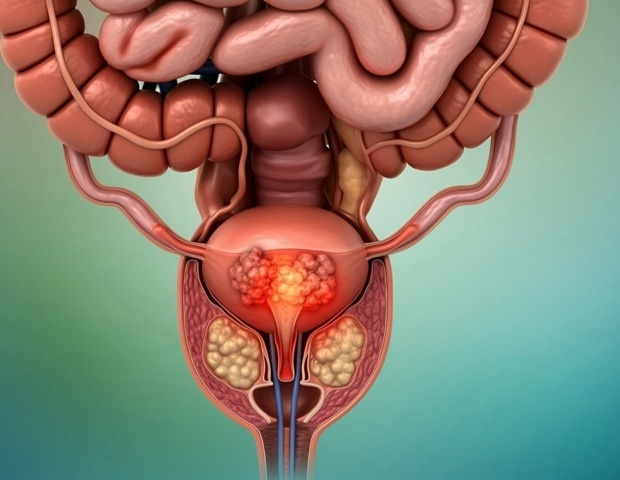
A bout of the common bladder infection, cystitis, may signal the presence of urogenital cancers – which affect parts of the body involved in reproduction and excretion – in middle-aged adults, according to research published in BMJ Public Health.
The risks seem to be especially high within three months of infection, suggesting acute cystitis might be a useful clinical marker for urogenital cancer. Men appear to be at greater risk than women,the findings indicate.
Researchers mined several national population and health registers and primary healthcare data sources, containing individual-level data on all people living in Sweden from 1997 to the end of 2018, analyzing data from 1,668,371 men and 1,889,211 women, 605,557 of whom were diagnosed with acute cystitis for the first time.
Over an average 15-year monitoring period, 257,026 (just over 7%) people were diagnosed with urogenital cancer, with prostate cancer being the most common (62%), followed by bladder cancer (16.5%) and endometrial cancer (10%).
Cystitis preceded a cancer diagnosis in 24,137 people – almost 9.5% of all those diagnosed with cancer during the study period. The average age at diagnosis was 76. The risks of a urogenital cancer diagnosis were heightened across all age groups in those who had experienced cystitis, peaking within three months of infection, especially concerning prostate and bladder cancers, but persisting over several years for most cancers.
Specifically, the risk of prostate cancer was seven times higher within three months of infection, translating to approximately 551 excess cases per 10,000 person-years. The risk of bladder cancer was 3.5 times higher in men and more than three times higher in women with cystitis compared to those without. These elevated risks corresponded to around 40 and nearly 8 excess cases per 10,000 person-years respectively. Higher risks were also observed for gynecological cancers.
Okay,here’s a breakdown of the key information from the provided text,organized for clarity and potential use in answering questions or summarizing the content.
Recent Study Links Urinary Tract Infections to increased risk of Urogenital Cancers
The Emerging Connection: UTIs and Cancer Risk
recent research is highlighting a concerning link between frequent urinary tract infections (UTIs) and an elevated risk of developing various urogenital cancers,including bladder cancer,kidney cancer,and even,to a lesser extent,prostate cancer in men. While a UTI itself isn’t cancerous, chronic inflammation and repeated infections appear to contribute to cellular changes that can potentially lead to malignancy. This isn’t a cause-and-effect relationship in every case, but the correlation is becoming increasingly clear, prompting further examination into preventative measures and early detection strategies. Understanding the nuances of this connection is crucial for both patients and healthcare providers.
Understanding the Inflammatory Pathway
The core of this link lies in chronic inflammation.Each UTI triggers an inflammatory response in the urinary tract. While the body typically resolves this inflammation, repeated infections can lead to persistent, low-grade inflammation. This prolonged inflammation can:
* Damage DNA: Chronic inflammation generates reactive oxygen species (ROS) that can damage DNA, increasing the risk of mutations.
* Promote Cell Proliferation: Inflammation stimulates cell growth and division, potentially allowing mutated cells to proliferate unchecked.
* Suppress Immune Response: Long-term inflammation can weaken the local immune response, making it harder for the body to identify and eliminate cancerous cells.
* Alter the Microbiome: Frequent antibiotic use for UTI treatment can disrupt the natural gut microbiome and vaginal microbiome (in women), potentially impacting immune function and increasing susceptibility to further infections and cancer advancement.
Specific Cancers and UTI Correlation
The strength of the association varies depending on the type of urogenital cancer:
* Bladder Cancer: This shows the strongest link. Studies have demonstrated a substantially higher risk of bladder cancer in individuals with a history of recurrent UTIs. The chronic irritation of the bladder lining is believed to be a key factor.Specific strains of E. coli, a common UTI causing bacteria, produce toxins that can contribute to bladder cell damage.
* Kidney Cancer: While the connection isn’t as strong as with bladder cancer, research suggests a moderate increase in kidney cancer risk among those with frequent UTIs, particularly pyelonephritis (kidney infection). Inflammation within the kidney tissue is thought to play a role.
* Prostate Cancer: The link to prostate cancer is less definitive, but some studies indicate that chronic prostatitis (inflammation of the prostate), frequently enough caused by bacterial infections, might potentially be associated with a slightly increased risk.
* Urethral Cancer: Rare,but emerging data suggests a possible correlation,particularly in women with chronic urethritis (inflammation of the urethra) stemming from repeated UTIs.
Identifying Risk Factors & Populations
Certain individuals are at higher risk of both frequent utis and subsequent urogenital cancer:
* Women: Women are more prone to UTIs due to their shorter urethra, making it easier for bacteria to reach the bladder.
* Older Adults: Weakened immune systems and underlying medical conditions increase susceptibility to both UTIs and cancer.
* Individuals with Catheters: Long-term catheter use significantly increases the risk of UTIs and associated complications.
* People with Urinary Tract Abnormalities: Structural abnormalities can predispose individuals to recurrent infections.
* Individuals with Compromised Immune Systems: Conditions like diabetes, HIV/AIDS, or immunosuppressant medications weaken the body’s defenses.
* Smokers: Smoking is a known risk factor for both UTIs and bladder cancer.
Diagnostic Approaches & Early Detection
Given the potential link, proactive monitoring is essential. This includes:
- Regular Medical check-ups: Especially for individuals with a history of recurrent UTIs.
- Urine Cytology: A simple test that examines urine for abnormal cells. Useful for bladder cancer screening.
- Imaging Studies: CT scans or MRIs may be recommended if there are concerning symptoms or a high risk profile.
- Cystoscopy: A procedure were a thin,flexible tube with a camera is inserted into the bladder to visualize the lining. Considered the gold standard for bladder cancer diagnosis.
- PSA Testing (for men): Prostate-Specific Antigen testing can help detect potential prostate cancer, although it’s not solely indicative of cancer.
Prevention Strategies: Reducing UTI Risk
Reducing the frequency of UTIs is a key preventative measure. Effective strategies include:
* Hydration: Drinking plenty of water helps flush bacteria from the urinary tract.
* Proper Hygiene: Wiping from front to back after using the toilet.
* Cranberry Products: While research is mixed, some studies suggest cranberry juice or supplements may help prevent UTIs by preventing bacteria from adhering to the bladder wall.Note: Consult with your doctor before taking supplements, especially if you are on blood thinners.
* Probiotics: Supporting a healthy gut microbiome and vaginal microbiome with probiotics may help prevent infections.
* Post-Coital Voiding: Urinating after sexual activity can help flush out bacteria.
* Avoid Irritants: Limit caffeine, alcohol, and spicy foods, which can irritate the bladder.
* D-Mannose: A naturally occurring sugar that can prevent E. coli from adhering to the urinary tract walls.
Case Study: Recurrent UTIs Leading to Early Bladder Cancer Detection
A 62-year-old female patient presented with a 10-year history of recurrent UTIs, averaging 3-4 infections per year. Despite multiple courses of antibiotics,infections persisted. During a routine follow-up prompted by another UTI, a urine cytology test revealed abnormal cells.Further investigation with a cystoscopy confirmed early-stage bladder cancer. Early detection, facilitated by the patient’s history of recurrent UTIs and proactive screening, allowed for successful treatment and a positive prognosis. This case highlights the importance of investigating persistent UTI symptoms beyond simply treating the infection.
The Role of antibiotic Stewardship
While antibiotics are essential for treating UTIs, overuse can contribute to antibiotic resistance and disrupt the microbiome. Antibiotic stewardship programs aim to optimize antibiotic use by:
* using antibiotics only when necessary.
* Selecting the most appropriate antibiotic for the specific infection.
* Using the shortest effective course of antibiotics.
* Promoting preventative measures to reduce UTI incidence.
Future Research Directions
Ongoing research is focused on:
* Identifying specific bacterial strains and toxins that contribute to cancer development.
* Developing novel preventative strategies, including vaccines and microbiome-based therapies.
* Improving early detection methods for urogenital cancers in high-risk individuals.
* Investigating the long-term effects of antibiotic use on the microbiome and cancer risk.
Disclaimer: This article provides general information and should not be considered medical advice. Always consult with a qualified healthcare professional for diagnosis and treatment of any medical condition.
Keywords: UTI,urinary tract infection,bladder cancer,kidney cancer,prostate cancer,urogenital cancer,inflammation,antibiotics,microbiome,cyst