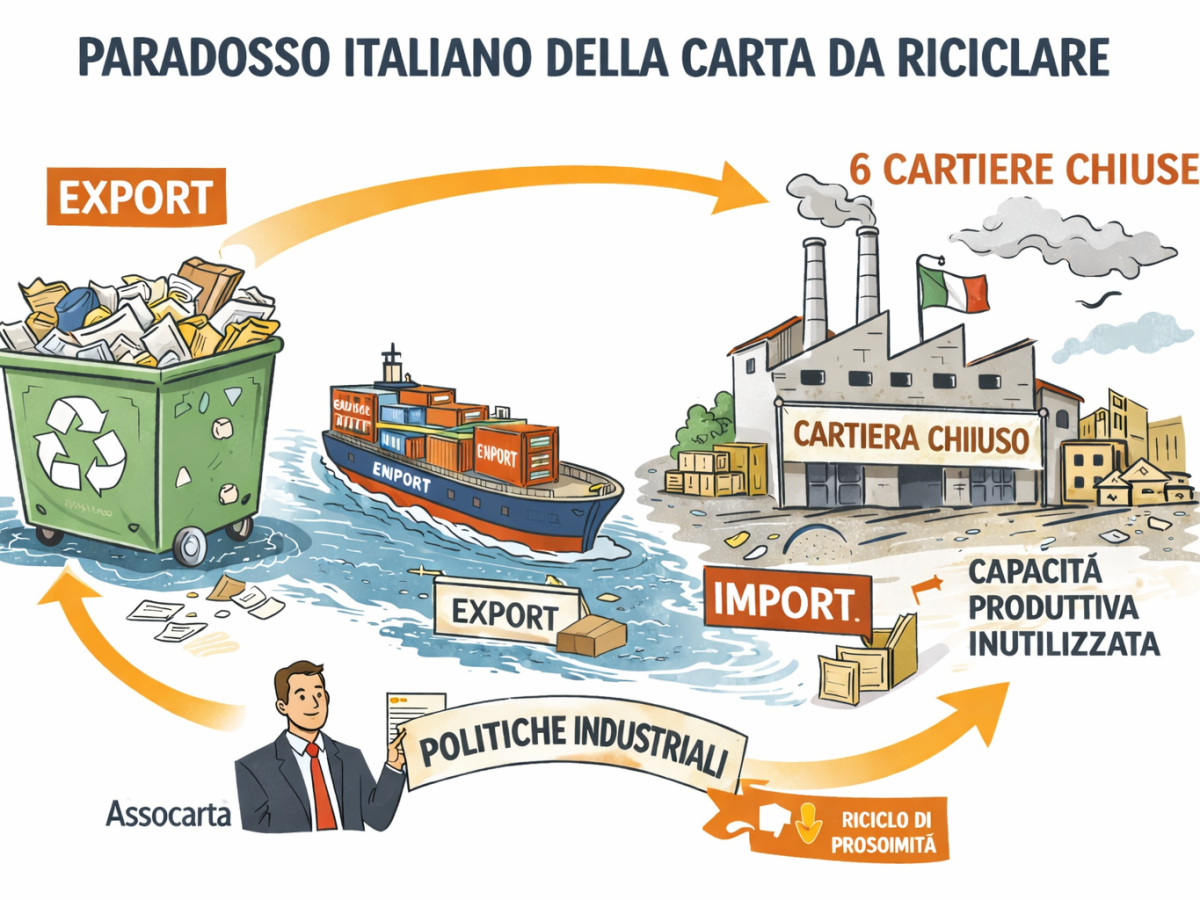
Lorenzo Poli (Assocarta): «Italy exports a quarter of the paper collected, canceling the environmental benefits of recycling. Industrial policies are needed to relaunch local recycling”
Italy is a world champion of paper recycling. It collects well, recycles better and over the years has built one of the most advanced industrial supply chains in Europe. But this record today coexists with an unprecedented alarm signal: «As of 2025, the operation of 6 out of 150 paper plants has ceased, after more than a decade in which the sector had practically never closed a plant». This, he warns Lorenzo Polipresident of Assocarta«is the most obvious symptom of a loss of structural competitiveness». Two of these plants could also reopen, but the current crisis is reflected in a paradox more and more marked: about a quarter of the paper collected in Italy goes abroad, even though the national industrial capacity would be sufficient to absorb it.
The numbers make visible a distortion that has progressively worsened in recent years. Every year Italy exports on average over 1.7 million tonnes of paper for recyclingjust under 25% of the national collection, while at the same time it imports paper and cardboard for packaging produced with the same raw material, but transformed elsewhere, with greater added value. «In practice», summarizes Poli, «we export a poor raw material and re-import a rich finished product. It’s the exact opposite of what a manufacturing country should do.”
The problem, explains the president of Assocarta, is not the lack of plants or the market. «We have everything: the raw material, the demand and the production capacity. The Italian demand for packaging paper could be satisfied entirely by national factories.” Nevertheless paper mills operate at 70–75% capacity on average. «But for a continuous cycle industry it would be necessary to stay steadily between 90 and 95%. Below that threshold there is no balance: the raw material remains unused and the system self-balances by exporting it.”
Energy costs and environmental management slow down competitiveness
Table of Contents
- 1. Energy costs and environmental management slow down competitiveness
- 2. Export to Asia
- 3. Why are European paper mills closing while india is reprocessing our waste paper into finished products?
- 4. Paper,the Paradox of Recycling: “6 Paper Mills Closed,While India Resells Us the Products Made From Our Waste Paper”
- 5. The Journey of Recycled Paper: From Bin to… India?
- 6. Why Are European Mills Closing?
- 7. The Economic Impact: A Lost Opportunity
- 8. The Role of Government and Policy
- 9. Beyond Recycling: Reducing Paper Consumption
- 10. Case Study: germany’s Approach
- 11. The future of Paper Recycling
At the basis of this loss of competitiveness there are above all two factors: the cost of energy and the management of environmental aspects. «In France, Germany or Spain», observes Poli, «paper mills manage to sell even at ten euros less per ton or at discounts of 3%. Today they make all the difference.” On the environmental front, the comparison is equally penalizing. «In other European countries, pulp waste is exploited in waste-to-energy plants, producing energy. In Italy we often have to pay for them to be picked up and disposed of. We lose the resource and we lose value.”
The result is a growing flow abroad, especially towards Asia. The most recent data from the European industry federation shows that, between January and October 2025, European Union exports of paper for recycling to India increased by more than 60% compared to the same period of the previous year. “Asia is an opportunistic buyer,” warns Poli. «Today it is there, tomorrow it may not be there. We have already seen it with China: when imports closed, the market collapsed and prices went below zero.”
Export to Asia
Dependence on these external outlets exposes the system to industrial and environmental risks. «If tomorrow India made the same choice and we did not have sufficient plants or waste-to-energy plants», says Poli, «circularity would be seriously compromised».
According to a study commissioned by Assocarta, if Italy managed to internally recycle all the paper it exports today, the benefits would be immediate and measurable: sector productivity would increase by 27%, approximately 1,360 new jobs would be created and the GDP would grow by 1.4 billion euros per year. «Keeping value in the territory», underlines Poli, «also means reducing emissions: exporting paper to be recycled involves more CO₂ than recycling it in Italy».
In short, today the intersection between high costs and low prices is decimating the industry. Hence Assocarta’s appeal to the public decision-maker. «The first priority is to become truly European on the cost of energy» insists Poli. “If this issue is not resolved, any further discussion is useless.” Then we need an environmental assessment that rewards proximity. «If I collect and recycle in Italy, I must be rewarded: the impact is smaller and the plants are kept alive». Finally the Ets. «The taxation of third sector bodies made sense and gave results, but today it has become a generalized taxation that penalizes companies without producing further environmental benefits».
The final message is clear. «Italy doesn’t just have raw materials and plants», concludes Poli. «It also has a highly qualified workforce. Industrial policies must enhance it more. Otherwise we will continue to ship value abroad and buy it back at a high price. It’s a luxury we can no longer afford.”
Why are European paper mills closing while india is reprocessing our waste paper into finished products?
Paper,the Paradox of Recycling: “6 Paper Mills Closed,While India Resells Us the Products Made From Our Waste Paper”
The image is stark: overflowing recycling bins in European cities,diligently sorted by citizens,ultimately feeding a system where the processed materials return as finished products – often from overseas. Specifically, the troubling trend of exported waste paper being remade into goods, and then re-imported, while domestic paper mills shutter their doors.The recent closure of six paper mills across Spain, despite robust paper recycling rates, exemplifies this frustrating paradox. It begs the question: what’s happening with our recycled paper,and why isn’t it benefiting local economies?
The Journey of Recycled Paper: From Bin to… India?
For years,Europe has championed paper recycling. High collection rates – often exceeding 70% in many countries – create a considerable stream of recovered fiber. However, the infrastructure to process that fiber domestically hasn’t kept pace. This creates a reliance on export markets, primarily in Asia, and increasingly, India.
Here’s a simplified breakdown of the process:
- Collection: Households and businesses separate paper and cardboard for recycling.
- Sorting & Baling: Materials are collected, sorted by grade (newspaper, cardboard, mixed paper), and baled for transport.
- Export: Bales are shipped, often to India, China, and other Asian countries.
- Reprocessing: These countries have invested heavily in paper reprocessing infrastructure. The waste paper is pulped, de-inked, and turned into new paper products.
- Re-import: Finished goods – packaging, cardboard boxes, even some printing paper – are shipped back to Europe.
This circular flow sounds positive on the surface, but the economic implications are deeply concerning.
Why Are European Mills Closing?
the core issue isn’t a lack of recyclable paper; it’s a lack of economic viability for European paper mills. Several factors contribute to this:
* Lower Labor Costs: Asian mills, particularly in India, benefit from substantially lower labor costs, giving them a competitive edge.
* Investment in Infrastructure: Massive investment in modern paper recycling facilities in Asia has created a processing capacity that Europe struggles to match.
* Energy Costs: Europe’s higher energy costs further disadvantage domestic mills. Paper production is an energy-intensive process.
* regulatory Burden: Stricter environmental regulations in Europe, while beneficial can increase operating costs for mills.
* Global Paper Demand: Increasing global demand for paper and packaging, particularly from rapidly developing economies, puts pressure on supply chains and incentivizes export.
The Economic Impact: A Lost Opportunity
The closure of those six Spanish paper mills isn’t just a statistic; it represents a critically important loss of jobs, economic activity, and regional development. Each mill supported numerous direct and indirect employment opportunities – from forestry workers to logistics personnel.
Furthermore, the reliance on imported finished paper products weakens Europe’s supply chain resilience. Dependence on overseas manufacturing creates vulnerabilities to geopolitical instability, shipping disruptions (as seen during the pandemic), and fluctuating exchange rates.
The Role of Government and Policy
Addressing this paradox requires a multi-faceted approach involving government intervention and policy changes. Potential solutions include:
* Investment in Domestic Infrastructure: Providing financial incentives for European paper mills to upgrade their facilities and increase processing capacity.
* Subsidies & Tax Breaks: Offering subsidies or tax breaks to offset higher energy and labor costs for domestic mills.
* Stricter Export Controls: implementing stricter controls on the export of waste paper,potentially prioritizing domestic processing. This is a sensitive issue, as it could be seen as protectionist.
* Promoting Enduring Procurement: Encouraging government agencies and businesses to prioritize purchasing paper products made from recycled materials within Europe.
* Extended Producer Duty (EPR): strengthening EPR schemes to hold producers accountable for the end-of-life management of their paper packaging.
Beyond Recycling: Reducing Paper Consumption
While improving recycling infrastructure is crucial, it’s only part of the solution. Reducing overall paper consumption is equally critically important.
* Digital Transformation: Embrace digital alternatives to paper-based processes whenever possible.
* Sustainable Packaging: Encourage the use of sustainable packaging materials, such as compostable or biodegradable alternatives.
* Print wisely: Only print when necessary, and use both sides of the paper.
* Support Paperless Initiatives: Advocate for paperless billing, statements, and dialog.
Case Study: germany’s Approach
Germany has taken a proactive approach to strengthening its domestic paper recycling industry. Through a combination of investment in modern facilities, stringent environmental regulations, and a strong focus on EPR, Germany has managed to maintain a robust domestic paper recycling sector and minimize its reliance on exports. While not a perfect system, it serves as a valuable model for other European countries.
The future of Paper Recycling
the paradox of paper recycling highlights the complexities of the global waste management system.Simply collecting and sorting materials isn’t enough.