“`html
Cancer Survivors May face Lower Alzheimer’s Risk, New Research Suggests
Table of Contents
- 1. Cancer Survivors May face Lower Alzheimer’s Risk, New Research Suggests
- 2. The alzheimer’s Puzzle: Beyond Amyloid Plaques
- 3. A Delicate Balance: The Immune System’s Dual Role
- 4. Historical Insights and Recent Findings
- 5. How does surviving cancer lower the risk of developing AlzheimerS disease?
- 6. Cancer Survivors Show Reduced Alzheimer Risk, New Study Reveals Immune Link
- 7. The Immune System: A Key Player
- 8. How Cancer Treatment Might Offer Protection
- 9. The Western Pacific Region & Cancer Incidence
- 10. Understanding the Specifics: What the Research Shows
- 11. Benefits of an Active Immune System for Brain Health
- 12. Practical Tips for Boosting Your Immune system
- 13. The Future of Research
Groundbreaking new research indicates a surprising connection between Cancer and Alzheimer’s disease: individuals with a history of Cancer appear to have a reduced likelihood of developing alzheimer’s. The findings,published recently in the journal Cell,point to the Immune system as a key factor in this complex relationship,offering potential new avenues for therapeutic interventions.
The alzheimer’s Puzzle: Beyond Amyloid Plaques
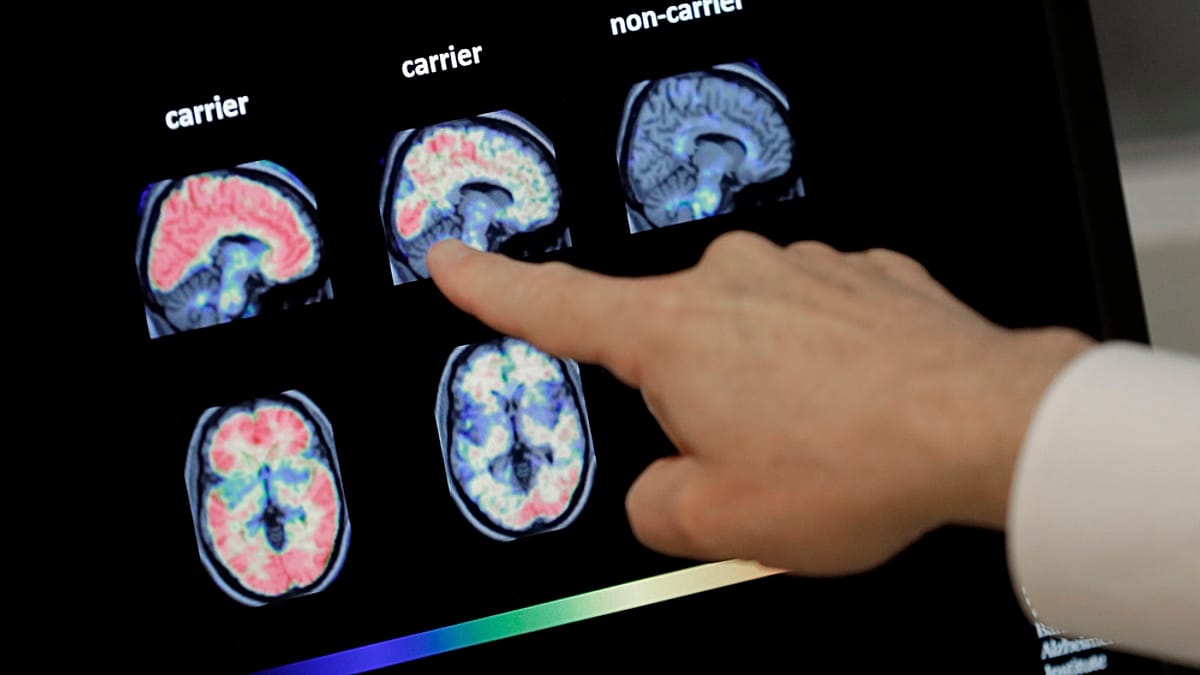
Alzheimer’s disease, the most prevalent form of dementia, impacts millions worldwide. It is traditionally characterized by the buildup of amyloid and tau proteins in the brain, leading to Neuron damage and progressive cognitive decline. According to the Alzheimer’s Association, over 6.7 million Americans are currently living with Alzheimer’s in 2024. However, emerging research challenges the notion that these protein deposits are the sole cause of the disease.
Scientists at New York University suggest that the body’s Immune response to these plaques – rather than the plaques themselves – may be the primary driver of brain damage. “some individuals can live with amyloid plaques without exhibiting symptoms,” explains Jordan Weiss, a longevity researcher at New York University. “The Immune system appears to be a crucial component in determining disease progress.”
A Delicate Balance: The Immune System’s Dual Role
The study reveals a captivating interplay between biological processes involved in both Cancer and Alzheimer’s. Researchers found that these two conditions frequently enough exist on opposing ends of the same spectrum. Accelerated biological activity may contribute to Cancer development, while diminished activity could increase the risk of Alzheimer’s.
This delicate balance hinges on the Immune system’s ability to regulate itself and manage protein accumulation. An overactive Immune system can trigger inflammation and Nerve cell destruction, potentially contributing to Alzheimer’s, while a weakened Immune response can allow Cancer cells to proliferate unchecked. The body’s method of protein clearance also plays a crucial role; too much removal can harm essential proteins, while insufficient clearance leads to hazardous buildup in the brain.
Historical Insights and Recent Findings
The link between the two diseases isn’t entirely new. A 2012 study initially suggested that Cancer survivors demonstrated a reduced risk of Alzheimer’s, and conversely, individuals with Alzheimer’s were less likely to receive a Cancer diagnosis. More recent, large-scale research reinforces this connection.
A comprehensive analysis of data from over three million people, conducted in 2024, revealed a 25% reduction in dementia risk among Cancer survivors. Furthermore, a 2025 study highlighted a correlation between the APOE gene – a known Alzheimer’s risk factor – and a decreased risk of Cancer.
| Study Year | Key Finding | Sample Size |
|---|---|---|