The Italian Medicines Agency has extended the reimbursement of liso-cel to those more “difficult” patients who thus have a greater chance of recovery
One is also available in Italy new therapy for patients with lymphoma who are refractory (i.e. do not respond) to first-line chemo-immunotherapy or who, after finishing it, have a recurrence within 12 months.
The Italian Medicines Agency (Aifa) has, in fact, approved the reimbursement of cell therapy with CAR-T, lisocabtagene maraleucel (liso-cel) for the treatment of adults with diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma (HGBCL), Primary mediastinal large B-cell lymphoma (PMBCL) e follicular lymphoma grade 3B (FL3B). These are aggressive pathologies, which often tend to recur and the new treatment has proven to increase the chances of permanent recovery.
Aggressive non-Hodgkin lymphomas
Table of Contents
- 1. Aggressive non-Hodgkin lymphomas
- 2. The study: this improves the response of patients
- 3. A “revolution” in cancer treatment
- 4. What are the eligibility criteria for CAR‑T therapy in lymphoma patients?
- 5. Lymphomas & Beyond First-Line Treatment: A New CAR-T Therapy option
- 6. Understanding CAR-T Cell Therapy: A Personalized approach
- 7. Wich Lymphomas Respond to CAR-T Therapy?
- 8. New Developments: Beyond CD19 Targeting
- 9. Managing Side Effects: A Critical Component of Care
- 10. Real-world Impact: Patient stories & Emerging Data
- 11. Accessing CAR-T Therapy: What to Expect
Diffuse large B-cell lymphoma, the most common form of non-Hodgkin lymphomarepresents approximately 30% of all aggressive lymphomas, which have a more rapid clinical course and require timely treatment. It is a blood cancer characterized by rapid growth of B lymphocytes, a type of white blood cell (immune system cell), which is diagnosed around 13,200 Italians every year.
High-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma (which increasingly occurs in adolescents and young adults), and grade 3B follicular lymphoma are also aggressive forms of non-Hodgkin’s lymphoma. «Lymphomas are treatable and in many cases curable diseases, thanks to availability of numerous therapies which, in recent years, have changed the treatment scenario – he explains Paolo Corradini, professor of Hematology at the University of Milan and director of the Division of Hematology and Bone Marrow Transplant at the IRCCS Foundation National Cancer Institute of Milan -. About 70% of patients with aggressive lymphomas can recover, and people with indolent lymphomas can achieve very long disease-free survival, up to 15 years.”
The approval of this this new CAR-T, lisocabtagene maraleucel, specifically affects those patients who do not benefit from first-line chemo-immunotherapy because it has no effect on their tumor (they are refractory) or because they suffered a relapse quickly.
The study: this improves the response of patients
Nello studio TRANSFORM, involving 184 patients with diffuse large B-cell lymphoma, the median event-free survival, after a median follow-up of almost 34 months, reached 29.5 months with liso-cel compared to 2.4 months with standard of careconsisting of chemotherapy followed by autologous transplant. At three years old progression-free survival at 51% compared to 26.5% and overall survival at 63% versus 52%.
Liso-cel has already been available in Italy since June 2024 for patients who have relapsed or are refractory to treatment after two or more lines of systemic therapy. «The extension of the reimbursement of liso-cel by Aifa concerns patients who relapse after the first line: in this way, treatment with cell therapy is brought forward – he clarifies Corradini -. In the TRANSFORM trial, patients with diffuse large B-cell lymphoma treated with liso-cel continue to show improved event-free survival and progression-free survival. Not only that. Improves complete response ratewhich in an aggressive disease such as large B-cell lymphoma is the prerequisite for recovery, was equal to 74% compared to 43%: this increase translates into an improvement in survival”.
A “revolution” in cancer treatment
The CAR-T therapy it is one of the greatest achievements of scientific research and one of the greatest “revolutions” in cancer treatment: has changed in the space of a few years the prospects for patients with blood cancers for whom “there was nothing left to do” (they had failed to achieve results with all other available treatments) and that now they even can heal and get your life back to the fullest. There are several CAR-Ts available todaywhich consist of a genetic modification of the patient’s T lymphocytes, which are induced to eliminate the neoplastic cells once reinfused.
They are part of the so-called advanced therapies and specifically they are therapies partly cell phones, as they use the patient’s T lymphocytes, partly geneticas T lymphocytes are genetically modified.
«CAR-Ts are “chemo-free“, that is, they do not involve use in association with chemotherapy, with important advantages for patients – continues Corradini -. Furthermore, in patients refractory to chemotherapy, CAR-Ts can determine recovery when traditional treatments, such as chemotherapy, transplant or radiotherapy, do not offer advantages. In the TRANSFORM study, liso-cel has proven to be an effective, short-lasting treatment because a single infusion is enough, capable of guaranteeing a good quality of life and an improvement in treatment possibilities”.
«The significant progress made in recent years in the treatment of blood cancers, such as the use of CAR-T therapies, have profoundly changed the therapeutic path of many patients, opening up new perspectives even for those who until recently had limited options – conclude Giuseppe Toro, national president of AIL (Italian Association against Leukemia, Lymphoma and Myeloma) -. In this rapidly evolving scenario, it is essential that patients do not feel alone and can count on care that goes beyond the strictly clinical aspect. The needs of patients with lymphoma are complex and also concern psychological support, access to correct and certified medical information, orientation in treatment paths and constant assistance during all phases of the disease. AIL has always been committed to responding to these needs by offering concrete support through the network of volunteers, more than 17,000 throughout Italy, and the AIL accommodation homes, which allow patients and their families to face treatment with greater serenity, close to hematology centres”.
What are the eligibility criteria for CAR‑T therapy in lymphoma patients?
Lymphomas & Beyond First-Line Treatment: A New CAR-T Therapy option
for individuals battling lymphomas – cancers that originate in the lymphatic system – the treatment journey can be complex. While initial, or first-line, therapies like chemotherapy and radiation often prove effective, a significant number of patients unfortunately experiance relapse or find their disease unresponsive to these standard approaches. Fortunately, advancements in immunotherapy are offering renewed hope, particularly wiht the emergence of new CAR-T cell therapies.
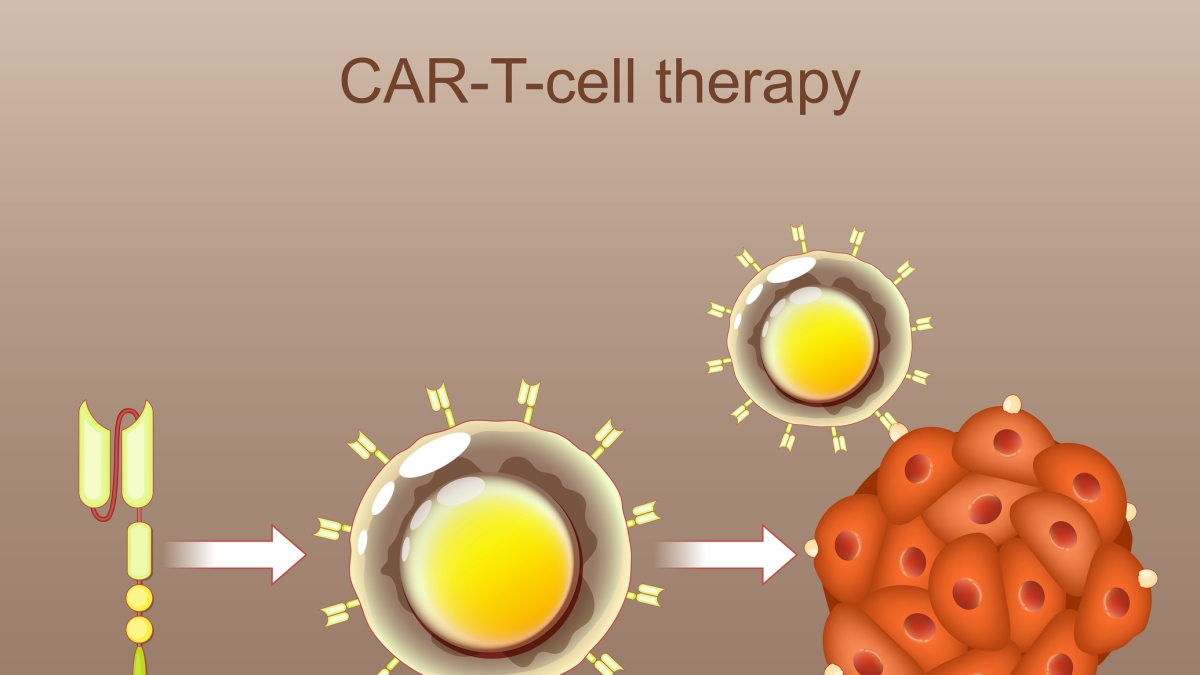
Understanding CAR-T Cell Therapy: A Personalized approach
Chimeric Antigen Receptor (CAR) T-cell therapy isn’t a one-size-fits-all treatment. It’s a highly personalized form of immunotherapy that harnesses the power of your own immune system to fight cancer. Here’s a breakdown of the process:
- T-Cell Collection: T cells, a type of white blood cell crucial for immune function, are collected from the patient’s blood. This process, called leukapheresis, is similar to dialysis.
- Genetic Modification: In a specialized laboratory, these T cells are genetically engineered to express a CAR. This CAR is designed to recognize a specific protein, or antigen, found on the surface of lymphoma cells. Currently approved CAR-T therapies primarily target the CD19 protein, common in many B-cell lymphomas.
- Cell Expansion: The modified CAR T cells are then grown and multiplied in the lab to create a large enough dose for treatment.
- Infusion: The expanded CAR T cells are infused back into the patient’s bloodstream. These “re-engineered” cells now actively seek out and destroy lymphoma cells expressing the targeted antigen.
Wich Lymphomas Respond to CAR-T Therapy?
While CAR-T therapy shows promise across various lymphoma subtypes, it’s currently most frequently used for:
* Diffuse Large B-cell Lymphoma (DLBCL): Particularly for patients who have relapsed after, or are refractory to, two or more lines of systemic therapy.
* Follicular Lymphoma (FL): Increasingly used for patients with relapsed/refractory FL, especially those with high-grade transformation.
* Mantle Cell Lymphoma (MCL): Approved CAR-T therapies are available for MCL patients who have tired other treatment options.
* Primary Mediastinal Large B-cell lymphoma (PMLBL): A more aggressive subtype of DLBCL, CAR-T therapy is demonstrating encouraging results.
The eligibility criteria are stringent and depend on the specific CAR-T product and the patient’s overall health. A thorough evaluation by a hematologist-oncologist experienced in cellular therapies is essential.
New Developments: Beyond CD19 Targeting
The field of CAR-T therapy is rapidly evolving. Researchers are actively exploring therapies that target antigens beyond CD19. This is crucial because some lymphomas can develop resistance by losing CD19 expression.
* Dual-Targeting CAR-T Cells: these innovative therapies are engineered to recognize two different antigens simultaneously, reducing the risk of resistance.
* CAR-T Cells Targeting Novel Antigens: Research is focused on identifying and targeting antigens uniquely expressed on lymphoma cells, minimizing off-target effects.
* “Armored” CAR-T cells: These cells are modified to enhance their persistence and effectiveness within the tumor microenvironment, overcoming immunosuppressive factors.
Managing Side Effects: A Critical Component of Care
CAR-T therapy, while powerful, isn’t without potential side effects. These can range from mild to severe and require careful monitoring and management.
* Cytokine Release Syndrome (CRS): A systemic inflammatory response triggered by the activated CAR T cells. Symptoms can include fever, fatigue, nausea, and difficulty breathing.Tocilizumab, a monoclonal antibody, is frequently enough used to manage severe CRS.
* Neurotoxicity: Neurological side effects, such as confusion, seizures, and speech difficulties, can occur. The exact mechanisms are still being investigated.
* B-cell Aplasia: Because many CAR-T therapies target CD19, thay can deplete healthy B cells, leading to temporary immune deficiency. Immunoglobulin replacement therapy might potentially be necessary.
* Infections: Suppressed immune function increases the risk of infections. Prophylactic antibiotics and antiviral medications are often prescribed.
Specialized centers with experience in CAR-T therapy have established protocols for managing these side effects effectively.
Real-world Impact: Patient stories & Emerging Data
The impact of CAR-T therapy is best illustrated through patient experiences. While individual results vary, many patients who have exhausted all other treatment options have achieved complete remission with CAR-T therapy, experiencing a significant improvement in their quality of life.
For example,a retrospective study published in The Lancet Oncology (2024) demonstrated a 54% overall response rate and a 38% complete response rate in patients with relapsed/refractory DLBCL treated with a novel CAR-T product targeting a different antigen than CD19. These findings highlight the potential of expanding CAR-T therapy beyond traditional targets.
Accessing CAR-T Therapy: What to Expect
Accessing CAR-T therapy requires a referral to a certified treatment center. The process involves:
* Comprehensive Evaluation: to determine eligibility and assess overall health.
* Insurance Pre-Authorization: CAR-T therapy is expensive, and pre-authorization from insurance is typically required.
* Leukapheresis & Cell Manufacturing: This process can take several weeks.
* Lymphodepletion: Before CAR T-cell