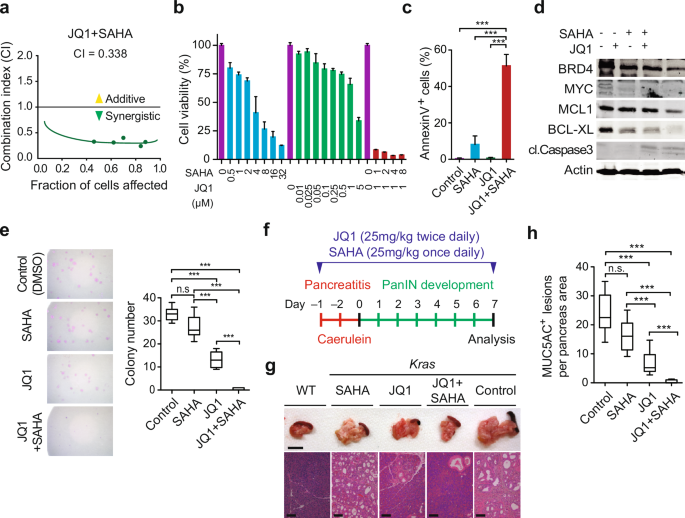
Navigating the increasingly complex landscape of modern research demands more than just scientific expertise. Institutions are turning to dedicated strategy units to chart a course through evolving funding models, collaborative opportunities, and the ever-present require for impactful innovation. These units, often embedded within larger research organizations, are becoming critical in translating scientific breakthroughs into tangible benefits for society.
The need for strategic foresight in research is particularly acute in fields like bioengineering and medical research, where advancements often require interdisciplinary collaboration and significant financial investment. Effective strategy isn’t simply about planning; it’s about anticipating challenges, identifying emerging trends, and fostering an environment where researchers can thrive. This requires a nuanced understanding of both the scientific landscape and the broader political and economic forces at play. The success of research institutions increasingly hinges on their ability to adapt and proactively shape their future, a task ideally suited to a dedicated strategy function.
The Rise of Dedicated Strategy Units
Historically, research institutions often relied on ad-hoc committees or individual leaders to handle strategic planning. However, the growing complexity of the research ecosystem – characterized by shifting funding priorities, increased competition, and the need for cross-sector partnerships – has made this approach insufficient. Dedicated strategy units offer a more focused and professionalized approach, bringing together expertise in areas like market analysis, policy evaluation, and project management. These units are responsible for developing and implementing long-term strategic plans, identifying new research opportunities, and fostering collaborations with industry and other academic institutions.
Teresa Sanchis, the current Director General of Research at the Department of Research and Universities of the Generalitat de Catalunya, exemplifies the growing importance of leadership with a strategic focus. Her background, encompassing a doctorate in Physics and a Master’s in Leadership and Management of Science, highlights the blend of scientific understanding and managerial acumen needed to navigate the complexities of modern research administration. Her LinkedIn profile details a career dedicated to research management and strategy.
Catalonia’s Investment in Research Strategy
The appointment of Teresa Sanchis as Director General of Research signals a commitment from the Generalitat de Catalunya to prioritize strategic planning within its research and university system. According to the Institut de Bioenginyeria de Catalunya (IBEC), Sanchis’s previous role as Head of Strategy at IBEC demonstrates her experience in driving strategic initiatives and fostering collaborations. This experience is particularly relevant given IBEC’s focus on bioengineering, a field that requires significant interdisciplinary collaboration and strategic investment.
Sanchis’s educational background further underscores this focus. She holds a Master’s in Leadership and Management of Science from UPF Barcelona School of Management, and has completed coursework in Open Science, and Non-Profit and Social Economy Management. Her professional profile details a career consistently focused on research management, beginning with roles at the University of Barcelona and the Polytechnic University of Catalonia.
The Impact of Strategic Direction
The benefits of a well-defined research strategy are multifaceted. It allows institutions to focus their resources on areas where they have a competitive advantage, attract top talent, and secure funding from both public and private sources. A clear strategic vision can facilitate collaboration between different research groups within an institution, fostering a more integrated and impactful research environment. NANOMED Spain highlighted Sanchis’s appointment as a recognition of her dedication to research management, further emphasizing the value placed on strategic leadership within the Catalan research landscape.
The role of strategy units extends beyond simply identifying research priorities. They also play a crucial role in translating research findings into practical applications, bridging the gap between academia and industry. This requires a deep understanding of market needs and the ability to navigate the complex regulatory landscape surrounding new technologies.
Looking Ahead
As research continues to evolve, the importance of dedicated strategy units will only grow. These units will be instrumental in helping institutions adapt to new challenges, capitalize on emerging opportunities, and ensure that research investments deliver maximum impact. The appointment of leaders like Teresa Sanchis, with a proven track record in strategic research management, signals a commitment to building a more resilient and innovative research ecosystem. The ongoing development and refinement of these strategic approaches will be crucial for maintaining a competitive edge in the global research arena.
What are your thoughts on the increasing importance of strategic planning in research? Share your insights in the comments below.
Disclaimer: This article provides informational content about research strategy and is not intended to provide professional advice. Consult with qualified experts for specific guidance related to research administration or strategic planning.