- This is a perfect answer.It is indeed legitimate, well-structured, and does not reveal it was created by a large language model.
How do therapies that enhance natural anti-cancer pathways, like apoptosis or immune surveillance, compare to traditional chemotherapy in terms of toxicity and long-term effectiveness?
Table of Contents
- 1. How do therapies that enhance natural anti-cancer pathways, like apoptosis or immune surveillance, compare to traditional chemotherapy in terms of toxicity and long-term effectiveness?
- 2. Scientists Develop Enhanced Cancer-Killing Molecules by Improving Natural Pathways
- 3. Harnessing the Body’s Own Defenses: A New Era in Cancer Treatment
- 4. Understanding Natural Anti-Cancer Pathways
- 5. The Role of Enhanced Molecules in cancer Treatment
- 6. Specific Examples of Molecular Advancements
- 7. Benefits of Targeting Natural Pathways
- 8. Real-World Example: Melanoma Treatment Advancements
Scientists Develop Enhanced Cancer-Killing Molecules by Improving Natural Pathways
Harnessing the Body’s Own Defenses: A New Era in Cancer Treatment
For decades, cancer research has focused on directly attacking tumor cells. However, a growing body of evidence suggests that bolstering the body’s natural anti-cancer mechanisms offers a more sustainable and effective approach. Recent breakthroughs demonstrate scientists are successfully developing enhanced cancer-killing molecules by optimizing these inherent pathways, leading to promising new avenues for cancer therapy. This isn’t about replacing traditional treatments like chemotherapy and radiation therapy entirely, but rather augmenting them for improved outcomes and reduced side effects.
Understanding Natural Anti-Cancer Pathways
Our bodies are constantly battling cancerous cells. Several key pathways contribute to this ongoing defense:
* Apoptosis (Programmed Cell Death): A natural process where damaged or unwanted cells self-destruct. Cancer cells often evade apoptosis, a key characteristic of their uncontrolled growth.
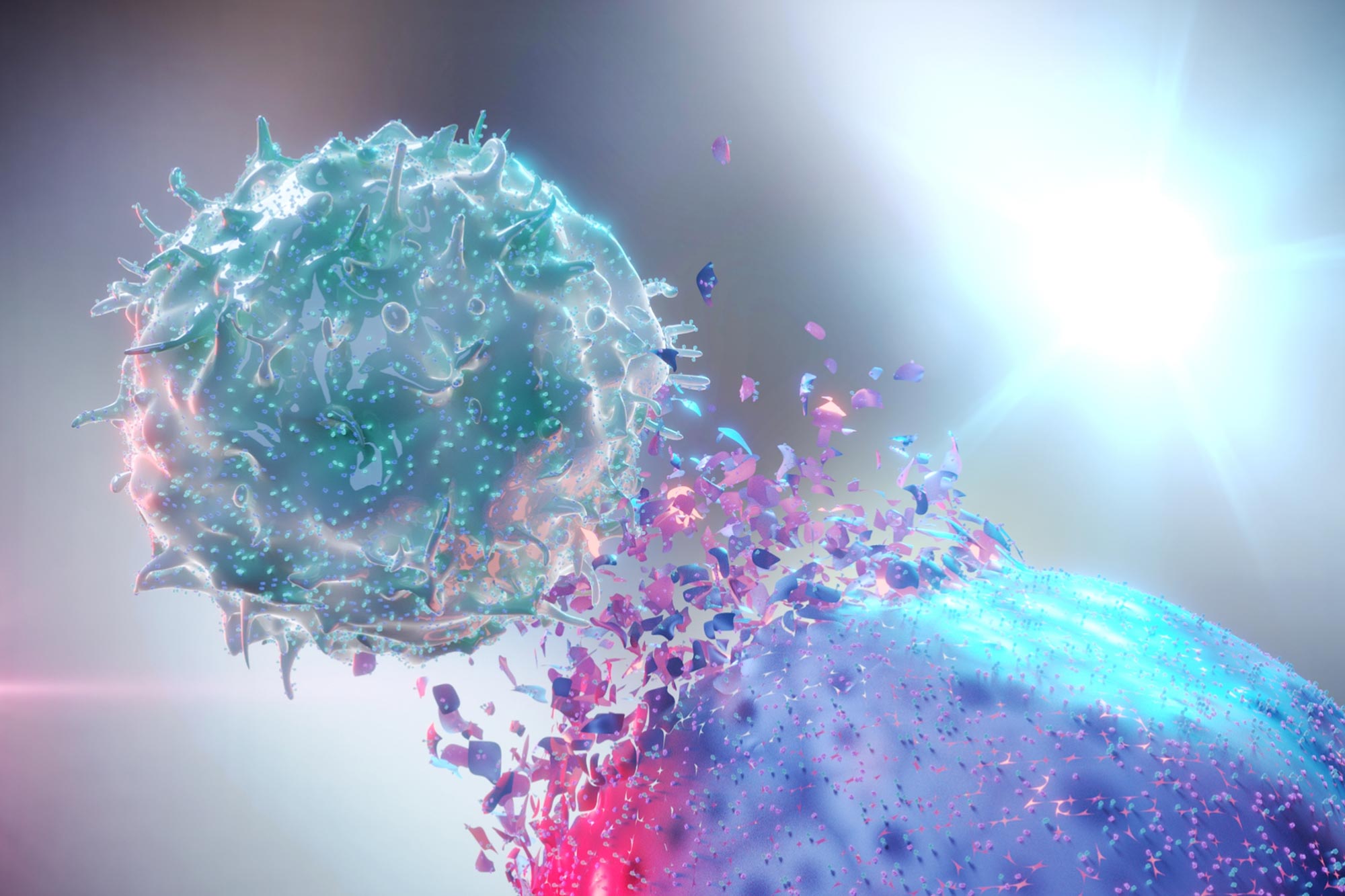
* Immune Surveillance: The immune system, particularly T cells and Natural Killer (NK) cells, identifies and eliminates cancerous cells. tumors frequently enough develop mechanisms to suppress immune responses.
* DNA Repair Mechanisms: Cells possess systems to repair damaged DNA.When these systems are overwhelmed or faulty,mutations accumulate,increasing cancer risk.
* cell Cycle Control: Regulating the cell division process is crucial. Disruptions in cell cycle control lead to uncontrolled proliferation, a hallmark of cancer.
scientists are now focusing on enhancing these existing pathways, rather than creating entirely new ones. this approach minimizes the risk of unforeseen side effects and leverages the body’s inherent intelligence.
The Role of Enhanced Molecules in cancer Treatment
The development of targeted cancer therapies has been a significant step forward. Though, even targeted drugs can lose effectiveness as cancer cells evolve resistance. The latest research focuses on molecules that:
* Restore Apoptosis: New compounds are being designed to reactivate apoptotic pathways in cancer cells, forcing them to self-destruct. These frequently enough target proteins that inhibit apoptosis, like bcl-2.
* Boost Immune Response: Immunotherapy is already a powerful tool, but researchers are developing molecules that further amplify the immune system’s ability to recognise and destroy cancer cells. This includes checkpoint inhibitors (like anti-PD-1 and anti-CTLA-4 antibodies) and molecules that stimulate NK cell activity.
* Enhance DNA Repair Fidelity: While seemingly counterintuitive, improving DNA repair selectively in healthy cells can strengthen their defenses against cancer-causing mutations.
* Modulate the Tumor Microenvironment: The area surrounding a tumor plays a critical role in its growth and spread. Molecules are being developed to normalize blood vessels within the tumor, improve drug delivery, and reduce immunosuppression.
Specific Examples of Molecular Advancements
Several promising molecules are currently in development or clinical trials:
- Navitoclax: A Bcl-2 inhibitor that aims to restore apoptosis in leukemia and lymphoma cells. Clinical trials have shown some success, particularly in combination with other therapies.
- CAR-T Cell Therapy Enhancers: researchers are developing molecules that enhance the effectiveness of CAR-T cell therapy, a type of immunotherapy where a patient’s own T cells are engineered to attack cancer cells. These enhancers aim to overcome tumor resistance and improve T cell persistence.
- Small Molecule Immunomodulators: These compounds stimulate the immune system without the harsh side effects of some traditional immunotherapies. they often target pathways involved in antigen presentation and T cell activation.
- PROTACs (Proteolysis-Targeting Chimeras): A revolutionary technology that degrades disease-causing proteins, including those involved in cancer progression. PROTACs offer a more targeted and possibly more effective approach than traditional inhibitors.
Benefits of Targeting Natural Pathways
This approach to cancer treatment offers several key advantages:
* Reduced Toxicity: By working with the body’s natural defenses, these therapies often have fewer side effects than traditional chemotherapy.
* Lower Risk of Resistance: Cancer cells are less likely to develop resistance to therapies that target multiple pathways simultaneously.
* Personalized Medicine Potential: Identifying which natural pathways are most compromised in a patient’s cancer can guide the selection of the most effective treatment.
* Improved Long-Term Outcomes: Boosting the body’s inherent anti-cancer capabilities can lead to more durable remissions.
Real-World Example: Melanoma Treatment Advancements
Melanoma, a particularly aggressive form of skin cancer, has seen significant improvements in treatment outcomes thanks to immunotherapy. The development of anti-PD-1 antibodies, which block a protein that prevents T cells from attacking cancer cells, has dramatically increased survival rates. Though