coverage with Evidence Development: CMS Approves T-TEER for Tricuspid Regurgitation Amidst Ongoing Debate
Table of Contents
- 1. coverage with Evidence Development: CMS Approves T-TEER for Tricuspid Regurgitation Amidst Ongoing Debate
- 2. What documentation is critical for submitting to CMS for pre-authorization of the TriClip procedure?
- 3. CMS Approves Tricuspid TEER Coverage – Conditions Apply
- 4. Understanding the Landmark Coverage Decision
- 5. Who Qualifies for CMS-Approved Tricuspid TEER?
- 6. The EVEREST II Trial & Evidence Supporting Coverage
- 7. Navigating the Coverage Process: What Patients Need to Know
- 8. Potential Benefits of Tricuspid TEER
Washington D.C. – The Centers for Medicare & Medicaid Services (CMS) has announced a decision to allow coverage for transcatheter edge-to-edge repair (T-TEER) for tricuspid regurgitation, but with a crucial caveat: “coverage with evidence development” (CED).This means that while patients can access the procedure, it will be contingent on their participation in specific clinical trials designed to further gather data on its effectiveness.The decision comes as the medical community grapples with differing interpretations of the existing evidence, primarily stemming from the pivotal TRILUMINATE trial. Earlier concerns had been raised that T-TEER had not demonstrated a “clinically meaningful benefit” and that TRILUMINATE failed to show a decrease in mortality, tricuspid valve surgeries, or overall hospitalizations when compared to traditional medical therapy.
However, CMS acknowledged that the evidence, while “insufficient,” is “promising enough” to warrant further investigation. “We believe the evidence while insufficient, is promising enough to allow coverage with evidence development,” the agency stated in its proclamation. The criteria for these new studies, which include tracking all-cause mortality and hospitalizations for a minimum of 24 months, are designed to strike a balance between generating robust evidence and ensuring patient access to potentially beneficial treatments.To be eligible for T-TEER under this CED pathway, patients must be experiencing symptomatic tricuspid regurgitation despite optimal medical management. Furthermore, they must be under the care of a multidisciplinary heart team, comprising a cardiac surgeon, an interventional cardiologist, a heart failure specialist, and an interventional echocardiographer.Dr. Shamir Mehta, a cardiologist at McMaster University in Hamilton, Ontario, Canada, and an investigator in the TRILUMINATE trial, defended the procedure’s merits. He highlighted that the trial did demonstrate improvements in patients’ quality of life, which he considers a significant outcome from the patient’s outlook. Dr. Mehta also pointed out that TRILUMINATE did observe a reduction in heart failure hospitalizations at the 2-year mark, suggesting that longer-term follow-up might reveal a correlation with reduced tricuspid regurgitation.
Dr. Mehta conceded that some criticisms of TRILUMINATE were valid,attributing the challenges partly to initial assumptions about the trial’s design. He explained that prior to TRILUMINATE, right heart failure and tricuspid regurgitation were relatively understudied. Consequently, the trial, along with the subsequent Triscend II trial, adopted the same event reduction paradigm used for left-sided heart failure. “We made an assumption that the same outcomes that we observed in mitral regurgitation would also apply to patients with right heart failure,” he stated, adding, “Maybe, the outcomes that we should be looking at with right-sided heart failure should be different.”
He further elaborated on the distinct presentations of left-sided versus right-sided heart failure. Patients with left-sided failure frequently enough experience pulmonary edema and require hospitalization for acute heart failure. In contrast, those with right-sided failure tend to accumulate fluid in the periphery and may be managed more frequently in outpatient clinics with diuretics, potentially leading to hospital admissions for complications like renal failure. “There are two types of heart failure, and they can present in very different ways,” Dr. Mehta emphasized.
The upcoming trials conducted under the CED process are expected to shed light on these distinctions, ultimately improving the understanding of when T-TEER is the appropriate treatment option for patients. “This is an evolving area and we’re learning more as we have more randomized trials,” Dr. Mehta concluded.
Dr. Mehta is also an investigator on the CLASP TR trials. Brian Owens contributed to this report as a freelance journalist based in New Brunswick, Canada.
CMS Approves Tricuspid TEER Coverage – Conditions Apply
Understanding the Landmark Coverage Decision
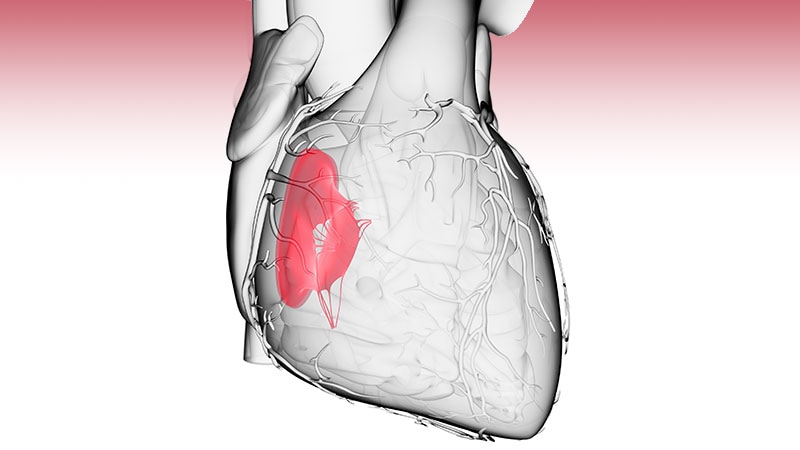
The Centers for Medicare & Medicaid Services (CMS) has recently approved coverage for Transcatheter Tricuspid edge-to-Edge Repair (TEER) wiht the Abbott TriClip™ device. This is a significant growth for patients suffering from tricuspid regurgitation (TR), a condition where the tricuspid valve doesn’t close properly, leading to blood leaking backward into the right atrium. For years, treatment options were limited, notably for those ineligible for or at high risk for open-heart surgery. This new coverage expands access to a possibly life-changing procedure. This article details the specifics of the CMS decision, eligibility criteria, and what it means for patients and healthcare providers.
Who Qualifies for CMS-Approved Tricuspid TEER?
CMS coverage isn’t worldwide. Specific criteria must be met for patients to be eligible for reimbursement for the TriClip procedure. these conditions are based on the clinical evidence presented in the pivotal EVEREST II trial and subsequent data. Key eligibility factors include:
Symptomatic Tricuspid Regurgitation: Patients must be experiencing symptoms related to TR, such as shortness of breath, fatigue, and swelling in the legs and abdomen. severity is often categorized using the New York Heart Association (NYHA) Functional Class.
Moderate to Severe TR: The degree of regurgitation must be at least moderate, and often severe, as persistent by echocardiography. Quantitative assessment of TR is crucial.
Right Heart Failure: Evidence of right heart failure, often indicated by elevated right atrial pressure and/or right ventricular enlargement, is a key requirement.
Suitable Anatomy: The tricuspid valve anatomy must be appropriate for the TriClip device.This is assessed through detailed imaging, including 3D echocardiography and potentially cardiac CT scans.
High Surgical Risk: Patients generally need to be deemed at high or prohibitive risk for conventional tricuspid valve surgery. This is often determined using a risk score calculator like the STS (Society of Thoracic Surgeons) score.
Life Expectancy: Patients should have a reasonable life expectancy to benefit from the procedure.
The EVEREST II Trial & Evidence Supporting Coverage
The CMS decision was heavily influenced by the results of the EVEREST II trial. This randomized controlled trial demonstrated the safety and efficacy of the TriClip device compared to medical therapy alone in patients with severe TR. Key findings included:
Reduced TR Severity: The TriClip device significantly reduced the severity of tricuspid regurgitation.
Improved Functional Capacity: Patients experienced improvements in their NYHA functional class, indicating better exercise tolerance and reduced symptoms.
Reduced Hospitalizations: The trial showed a trend towards fewer hospitalizations for heart failure in the TriClip group.
Safety Profile: The procedure was generally found to be safe, with manageable complications.
Ongoing real-world data collection and analysis will continue to inform CMS coverage policies.
Getting approved for Tricuspid TEER coverage requires a collaborative effort between the patient,cardiologist,and hospital. Here’s a breakdown of the process:
- Initial Evaluation: A cardiologist specializing in valvular heart disease will perform a thorough evaluation, including a physical exam, echocardiogram, and other necessary tests.
- Heart Team Discussion: The patient’s case will be discussed by a “heart team” – a multidisciplinary group of specialists including cardiologists, cardiac surgeons, and imaging specialists.
- Documentation & Pre-Authorization: Detailed documentation supporting the patient’s eligibility, including imaging reports, risk scores, and a statement of surgical ineligibility, must be submitted to CMS for pre-authorization. This is a critical step.
- CMS Review: CMS will review the submitted documentation and determine whether the patient meets the coverage criteria.
- Procedure & Follow-Up: If approved, the TriClip procedure can be scheduled. Regular follow-up appointments and echocardiograms are essential to monitor the patient’s progress.
Potential Benefits of Tricuspid TEER
Beyond the clinical improvements demonstrated in trials, Tricuspid TEER offers