Deadly Cough Syrup Linked to Child Deaths Prompts Nationwide Health Alert
Table of Contents
- 1. Deadly Cough Syrup Linked to Child Deaths Prompts Nationwide Health Alert
- 2. Toxic Substance Identified in Cough Syrup
- 3. Government Response and Crackdown
- 4. Nationwide Investigations and Inspections
- 5. Timeline of events and Affected Children
- 6. expert Analysis and Ongoing Investigations
- 7. Understanding the Dangers of Diethylene Glycol
- 8. Frequently asked Questions about the Cough syrup Crisis
- 9. What pharmaceutical quality control measures were lacking that allowed DEG contamination in Coldrif cough syrup?
- 10. Madhya Pradesh Bans Coldrif Cough Syrup Following Child Deaths – Toxic Chemical Confirmed
- 11. Urgent Ban and Investigation Details
- 12. Understanding Diethylene Glycol (DEG) Poisoning
- 13. The Madhya Pradesh Crisis: A Timeline of Events
- 14. Regulatory Response and Ongoing Investigations
- 15. Similar Incidents & Global Concerns
- 16. What Parents and Caregivers Should Do
- 17. The Role of pharmacovigilance
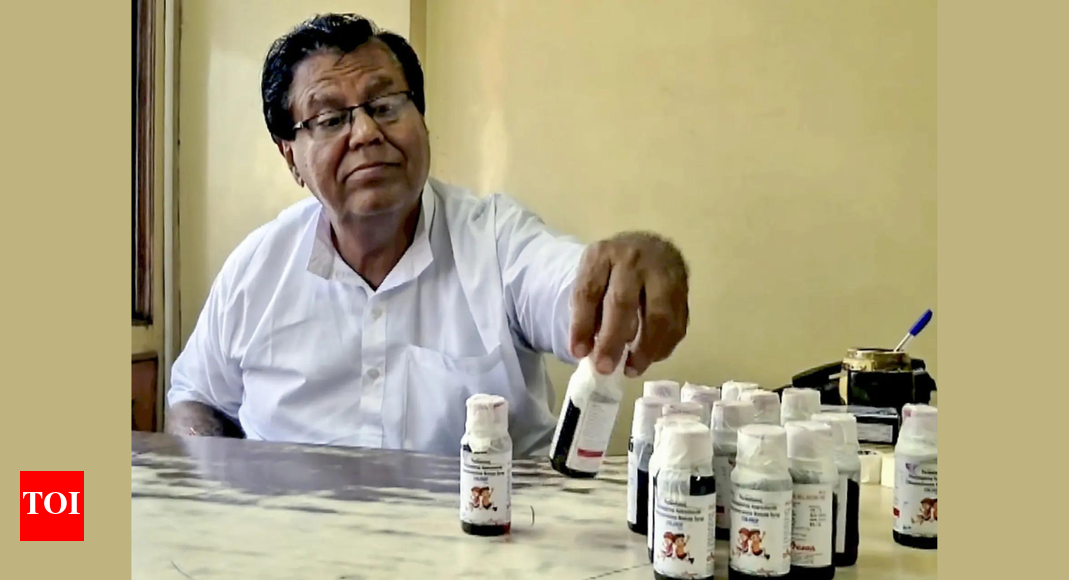
Bhopal/New Delhi – A growing health crisis is unfolding in India as a tainted cough syrup has been linked to the deaths of eleven children in the Chhindwara district of Madhya Pradesh.Recent laboratory tests have revealed the presence of a highly toxic industrial chemical, prompting immediate action from state and national health authorities.
Toxic Substance Identified in Cough Syrup
According to reports released Saturday,testing of the ‘Coldrif’ cough syrup revealed a 48.6% concentration of diethylene glycol (DEG). This hazardous substance, commonly used in antifreeze and brake fluids, is known to cause acute kidney failure and can be fatal if ingested. The revelation has triggered a widespread ban on the sale of Coldrif, manufactured by Sresan Pharmaceuticals, based in Tamil Nadu.
Government Response and Crackdown
The Madhya Pradesh government has initiated a thorough crackdown, ordering drug inspectors to seize all existing stocks of Coldrif and prevent further distribution. The ban has been extended to all pharmaceutical products manufactured by sresan Pharmaceuticals. Chief Minister Mohan Yadav announced a compensation of Rs 4 lakh (approximately $4,800 USD) for each affected family and pledged to cover the medical expenses for children still undergoing treatment.
Nationwide Investigations and Inspections
The crisis has triggered health alerts and investigations across multiple states in India. The Central Drug Standards Control Organisation (CDSCO) has launched risk-based inspections of drug manufacturing facilities in six states – Himachal Pradesh,Uttarakhand,Gujarat,Tamil Nadu,Madhya Pradesh,and Maharashtra. These inspections are specifically focused on companies producing cough syrups, antipyretics, and antibiotics.
Timeline of events and Affected Children
The deaths in Chhindwara have occurred over the past month, affecting children under the age of five. The initial symptoms included mild fever and colds, leading parents to seek treatment with over-the-counter cough syrups, including the now-banned Coldrif. As the children’s condition deteriorated, they developed acute kidney complications, and five are currently receiving care at a medical college hospital in Nagpur.
expert Analysis and Ongoing Investigations
A multidisciplinary team of experts from various national institutions, including the National Institute of Virology and the Indian Council of Medical Research, is conducting a thorough analysis of samples to determine the precise cause of the deaths. While initial tests by CDSCO and the Madhya Pradesh Food and Drugs Administration found some samples free of DEG and ethylene glycol, authorities clarified these tests did not include Coldrif, which remains under scrutiny.
Did You Know? The World Health Association (WHO) has issued multiple alerts regarding substandard and falsified medical products, particularly cough syrups, highlighting the global risk of contaminated pharmaceuticals.
| State | Action Taken | Focus of Examination |
|---|---|---|
| Madhya Pradesh | Banned Coldrif, seized stocks, compensation for families | Identifying source of contamination, treating affected children |
| Tamil Nadu | Manufacturer Sresan Pharmaceuticals under investigation | Production processes, quality control measures |
| Central Government | Launched nationwide inspections of drug manufacturers | Ensuring quality and safety of pharmaceutical products |
Understanding the Dangers of Diethylene Glycol
Diethylene glycol (DEG) is a toxic chemical compound that can cause severe health problems, even in small doses. It’s often used illegally as a cheaper choice to glycerol, a harmless ingredient in many medications. Symptoms of DEG poisoning include kidney failure, abdominal pain, vomiting, and neurological problems. The U.S. Food and Drug Administration (FDA) has established stringent guidelines to prevent DEG contamination in pharmaceutical products. Learn more about FDA safety alerts.
Pro Tip: Always purchase medications from reputable pharmacies and check for proper packaging and labeling.If you suspect a medication might potentially be contaminated, do not consume it and report it to your local health authorities.
Frequently asked Questions about the Cough syrup Crisis
- What is diethylene glycol and why is it dangerous? DEG is a toxic chemical used in industrial applications that can cause kidney failure and death when ingested.
- What is the government doing to address this issue? Authorities have banned the affected cough syrup, initiated investigations, and are inspecting pharmaceutical manufacturers nationwide.
- How can I protect my family from contaminated medications? Purchase medications from reputable sources and check for proper packaging.
- what are the symptoms of DEG poisoning? Symptoms include abdominal pain,vomiting,and kidney failure.
- Is this a widespread problem in India? While the current outbreak is concentrated in Madhya Pradesh, authorities are investigating similar cases in other states.
- What steps are being taken to prevent future incidents? The CDSCO is conducting risk-based inspections and strengthening quality control measures.
- What should people do if they have used Coldrif cough syrup? anyone who has used Coldrif cough syrup should seek medical advice immediately.
What are your thoughts on the need for stricter regulations in the pharmaceutical industry? Share your opinions in the comments below!
What pharmaceutical quality control measures were lacking that allowed DEG contamination in Coldrif cough syrup?
Madhya Pradesh Bans Coldrif Cough Syrup Following Child Deaths – Toxic Chemical Confirmed
Urgent Ban and Investigation Details
Following a tragic outbreak of illness and the confirmed deaths of eleven children in Madhya Pradesh, India, authorities have issued an immediate ban on the sale and distribution of Coldrif cough syrup. A preliminary report has identified the presence of a toxic chemical – diethylene glycol (DEG) – as the likely cause of the poisoning.This incident underscores the critical need for stringent pharmaceutical quality control and drug safety regulations within India.
* Date of Ban: October 4, 2025
* affected Product: Coldrif Cough Syrup
* Region Affected: Madhya Pradesh, India
* Confirmed Cause: Diethylene Glycol (DEG) contamination
* Fatalities: 11 children confirmed deceased. Numerous others hospitalized.
Understanding Diethylene Glycol (DEG) Poisoning
Diethylene glycol is a highly toxic industrial solvent that can cause severe kidney damage, neurological problems, and ultimately, death. Its presence in pharmaceutical products, notably syrups, is a recurring and devastating issue. symptoms of DEG poisoning can mimic other illnesses, making early diagnosis challenging.
* Initial Symptoms: Vomiting, abdominal pain, diarrhea, and dehydration.
* advanced symptoms: Acute kidney injury,neurological dysfunction (seizures,altered mental status),and respiratory failure.
* Vulnerable Populations: Children are particularly susceptible to the toxic effects of DEG due to their lower body weight and developing organ systems. Pediatric drug safety is paramount.
The Madhya Pradesh Crisis: A Timeline of Events
The crisis unfolded rapidly, prompting swift action from state and national health authorities.
- Initial Reports (Early October 2025): Hospitals in Madhya Pradesh began reporting a surge in cases of acute kidney injury among children, wiht a common link to the consumption of Coldrif cough syrup.
- Sample Collection & testing: Drug samples were immediately collected and sent to laboratories for analysis.
- DEG Confirmation (october 4, 2025): Laboratory tests confirmed the presence of diethylene glycol in the Coldrif cough syrup samples.
- State-Wide Ban: The Madhya pradesh government issued an immediate ban on the sale, distribution, and stock of Coldrif cough syrup.
- Investigation Launched: A high-level investigation has been launched to determine the source of the contamination and identify any potential negligence in the manufacturing or distribution process. Drug manufacturing standards are under scrutiny.
Regulatory Response and Ongoing Investigations
The Drugs Controller General of India (DCGI) has been informed and is coordinating with state authorities. The investigation will focus on several key areas:
* Manufacturer Accountability: Identifying the manufacturer of the contaminated Coldrif cough syrup and assessing their quality control procedures.
* Supply Chain Analysis: Tracing the supply chain of the raw materials used in the production of the syrup to pinpoint the source of the DEG contamination.
* Batch Recall: Initiating a nationwide recall of all affected batches of Coldrif cough syrup.
* Strengthening Quality Control: Implementing stricter quality control measures and increasing surveillance of pharmaceutical manufacturing facilities. Pharmaceutical regulation is being reviewed.
Similar Incidents & Global Concerns
This is not the first time DEG contamination has led to mass poisoning events. Several similar incidents have occurred in the past, raising serious concerns about pharmaceutical safety standards in developing countries.
* 2008 (China): Contaminated heparin, a blood thinner, caused deaths and injuries worldwide.
* 2009 (Nigeria): Paracetamol syrup contaminated with DEG resulted in the deaths of dozens of children.
* 2022 (Gambia): Cough syrups manufactured in India were linked to the deaths of nearly 70 children in Gambia, also due to DEG contamination. This prompted a WHO alert.
These past events highlight the need for international collaboration and robust global drug safety protocols.
What Parents and Caregivers Should Do
In light of this crisis,parents and caregivers should take the following precautions:
* Avoid Self-Medication: Do not administer cough syrup or any other medication to children without consulting a qualified healthcare professional.
* Verify Medication Authenticity: Ensure that all medications are purchased from reputable pharmacies and check for proper packaging and labeling.
* Report Suspicious Symptoms: If your child exhibits symptoms such as vomiting, abdominal pain, or decreased urination after taking cough syrup, seek immediate medical attention.
* Follow Official Advisories: Stay informed about official advisories and updates from health authorities regarding the coldrif cough syrup ban and any potential recalls. Public health alerts are crucial.
The Role of pharmacovigilance
Pharmacovigilance, the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related